Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Investigación & Desarrollo
versión impresa ISSN 1814-6333versión On-line ISSN 2518-4431
Inv. y Des. vol.18 no.1 Cochabamba 2018
http://dx.doi.org/10.23881/idupbo.018.1-1i
ARTÍCULOS – INGENIERÍAS
ELECTROCHEMICAL OXIDATION OF SYNTHETIC ORGANIC DYES BY FERRATE (VI), USING COMMERCIAL STEEL WOOL ELECTRODES
OXIDACIÓN ELECTROQUÍMICA DE TINTES SINTÉTICOS ORGÁNICOS MEDIANTE ION FERRATO (VI), USANDO ELECTRODOS DE LANA DE ACERO COMERCIAL
Ramiro Escalera Vásquez1, Uli Nicol Hosse Pastor1 and Pablo Marcelo Pérez García2
1Centro de Investigaciones en Procesos Industriales – CIPI, Universidad Privada Boliviana
2Utrecht University
(Recibido el 18 mayo 2018, aceptado para publicación el 16 de junio 2018)
ABSTRACT
This paper deals with the in-situ electrochemical oxidation of Reactive Black 5 (RB-5), Reactive Blue 19 (RB-19) and Allura Red AC (AR-AC), using commercial steel wool as electrodes. At the optimal conditions (18 V and NaOH 0.33M), the decolorization of RB-19 (anthraquinone-type dye) is much more rapid than those of azo dyes RB-5 and AR-AC. The reaction rates based on a first order reaction model were 0.134 min-1for RB-19, 0.043 min-1 for RB-5 and 0.028 min-1 for AR-AC. Color removal efficiencies were higher than 95% achieved in 120 min. The analyses of spectra of the three dyes in the visible region indicate a complete cleavage of both azo and quinoid chromophores. In the case of RB-19 no new absorption peaks occurred in the UV region, showing a partial oxidation of aromatic groups without the generation of intermediates. In case of both azo-type dyes RB-5 and AR-AC, formation/accumulation of intermediates followed by their partial oxidation may have occurred. All these observations indicate that the predominant mechanism for decolorization was the oxidation of the three dyes. We conclude that that the electrochemical oxidation by ferrate (VI), under low voltages and low NaOH concentrations, using commercial steel wool as electrodes is an efficient and cost-effective alternative for the decolorization of azo and anthraquinone type dyes. For future studies a COD analysis should be made in order to correlate the decolorization and the elimination of the organic load in the dye solutions.
Keywords: Ferrate (VI), Synthetic Organic Dyes, Electrochemical Oxidation, Decolorization.
RESUMEN
Este artículo trata de la oxidación electroquímica in situ de Reactive Black 5 (RB-5), Reactive Blue 19 (RB-19) y Allura Red AC (AR-AC), utilizando lana de acero comercial como electrodos. En las condiciones óptimas (18 V y NaOH 0,33 M), la decoloración de RB-19 (colorante de tipo antraquinona) es mucho más rápida que las de los colorantes azoicos RB-5 y AR-AC. Las velocidades de reacción basadas en un modelo de reacción de primer orden fueron 0,134 min-1 para RB-19, 0,043 min-1 para RB-5 y 0,028 min-1 para AR-AC. Las eficiencias de eliminación de color fueron superiores al 95% en 120 min. Los análisis de los espectros de los tres colorantes en la región visible indican una ruptura completa de los cromóforos tanto azoicos como quinoides. En el caso de RB-19 no se produjeron nuevos picos de absorción en la región UV, lo que muestra una oxidación parcial de grupos aromáticos sin la generación de compuestos intermedios. En el caso de los dos colorantes azoicos RB-5 y AR-AC, puede haber ocurrido la formación / acumulación de compuestos intermedios seguida de su oxidación parcial. Todas estas observaciones indican que el mecanismo predominante para la decoloración fue la oxidación de los tres tintes. Concluimos que la oxidación electroquímica por ferrato (VI), a voltajes bajos y bajas concentraciones de NaOH y utilizando lana de acero comercial como electrodos, es una alternativa eficiente y rentable para la decoloración de colorantes tipo azo y antraquinona. Para estudios futuros, se debe realizar un análisis de DQO para correlacionar la decoloración y la eliminación de la carga orgánica en las soluciones de tinte.
Palabras Clave: Ferrato (VI), Tintes Orgánicos Industriales, Oxidación Electroquímica, Remoción de Color.
1. INTRODUCTION
Until 2016, around 100 jean-dying small factories were actively operating in the city of Cochabamba, Bolivia[1]. The dying process includes several steps in which large amounts of clean water are required: removal of gums from the fabric using detergents, color addition, fixing, neutralizing with meta-bisulfides and brightening. Moreover, each of these steps is followed by washing.
The whole process generates large volumes of highly polluted wastewaters that are partially treated by simple solids screening and settling before their discharge to the sewage system. As a result, the quality of the effluents does not comply with Bolivian environmental regulations, especially regarding concentration levels of Chemical Oxygen Demand (COD), chloride, sulfur, suspended solids, total solid and total dissolved solids[1]. The color addition step requires high quantities of synthetic dyes (mainly azo and anthraquinone types) which contribute to high COD levels according to Zaconeta & Escalera[2]. These high concentrations of non-biodegradable matter and persistent color cause problems for living organisms in natural water bodies[3].
A variety of wastewater treatment processes have been proposed for Reactive Black 5 (RB-5), Reactive Blue 19 (RB-19) and Allura Red AC (AR-AC), which are extensively used by Bolivian textile industries. The processes, among others, include: microbial decolorization[4], biological anaerobic degradation[5], adsorption[6] [7] [8], electro-oxidation[9] [10] [11] [12], electro-coagulation[13], ozonation[14] [15], Fenton[16] [17], photo-Fenton[18], photo-Fenton UVA treatment through solar collectors[2], solar photo-electro-Fenton[12], photo-catalysis coupled by ultrasonic treatment[19] and ferrous-activated persulfate oxidation [20], each having different advantages and disadvantages.
Ferrate (VI) has received attention as a green alternative for the treatment of organically polluted industrial wastewaters due to its high oxidative potential and good coagulant properties[3] [21]. Concerning its application in the oxidation of synthetic dyes, the research has been focused mainly towards the degradation of azo dyes. Li et al. [22] and Xu et al. [23] used wet chemical synthesis to prepare a ferrate (VI)-hypochlorite composite solution (10% NaClO and Fe(NO3)3·9H2O in high basic media at 50-65 °C), for the oxidation of Orange II and Reactive brilliant red X-3B, respectively. Other authors used self-made and purchased solid potassium ferrate to prepare solutions at mildly acidic conditions (pH range of 4 – 7) for the treatment of Reactive brilliant red X-3B[24], Reactive Orange 16[25], Reactive Red 2BF[26] and Acidic Red-dye[27]. In contrast, the studies on the ferrate (VI) decolorization of RB-5, AR-AC and RB-19 have been scarce[28] [29]. In all cases, a ferrate (VI) solution was previously prepared or synthesized and then was dosed to the dye solution at adequate concentrations. In these studies, color removal percentage values were higher than 90% treating dye contents in the 25-200 ppm interval, during 15 – 60 min.
In the last decade, several researchers used the in-situ electrochemical approach to prepare ferrate (VI) solutions due to its advantages over the wet and dry chemical synthesis as it is simple, cost effective and does not require hypochlorite. A 97% color removal was achieved for a 50 ppm Methylene Blue solution in which samples were treated by in-situ electro-generated ferrate (VI) in basic aqueous solutions, using high purity iron plates[30]; dosing of ferrate (VI) solution was made immediately using a peristaltic pump. A 40 ppm dye solution of Acid yellow 36 was decolorized up to 94% by in-situ electrochemical generation of ferrate (VI) using boron-doped diamond (BBD) electrodes in acidic conditions[31]. In this case, of Fe2SO4 1 mM was dosed directly to the dye solution to synthesize ferrate (VI).
Nevertheless, as reported by Barisçi et al.[32], the decomposition rate of in-situ electro-synthesized ferrate (VI) depends on the initial ferrate (VI) ion concentration, alkalinity, pH of the solution, electrolyte type and temperature. Ferrate (VI) ion showed high stability at higher alkalinity (higher hydroxide concentration), lower temperatures (below 30°C), increasing ferrate (VI) ion concentration, pH in the range between 9.2 and 10 and NaOH as electrolyte. Its instability at low pH levels was explained to be caused by the speciation of ferrate (VI) in alkaline and acidic conditions; i.e. at alkaline conditions ferrate remains as a more stable FeO4-2, and a less stable HFeO4 - is predominant in mildly acidic conditions.
In sequential processes in which in-situ electrochemical synthesis of ferrate (VI) is followed by its dosage to dye solutions, the amount of sodium hydroxide added to the solution is usually high, causing significant increase in pH and sodium concentrations. This aspect can be a pitfall at the time of its application for textile wastewater treatment, due to environmental restrictions.
This paper deals with the in-situ electrochemical oxidation of Reactive Black 5, Reactive Blue 19 and Allura Red AC in which the electrochemical synthesis of ferrate (VI) and its dosage to dye solutions are carried out simultaneously. The study aims to prevent a ferrate degradation before the dosage and to reduce significantly the sodium concentration in the final treated wastewater.
2. MATERIALS AND METHODS
• General
The UV-vis spectra analyses were carried out using a Shimadzu UV-1601 spectrometer using quartz cells with 1 cm length. The organic dyes Reactive Blue 19 (RB-19), Reactive Black 5 (RB-5) and Allura Red AC (AR-AC) were commercial grade and were supplied by a local factory[2] . Technical grade NaOH pellets (93.43%) were used in the dye degradation process. All the reagents were used without further purification.
• Analytical measurements
The pH of all solutions were measured using a Hanna pH/ORP/DO meter model HI98196. following the procedure described by standard method 4500-H+ B.
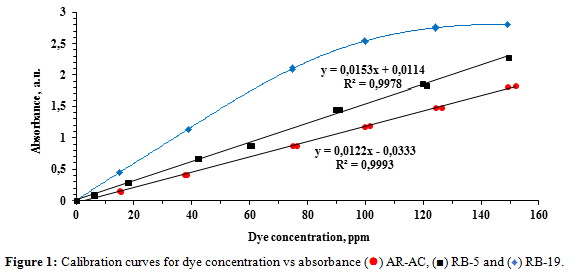
The characterization of the dyes and the wavelengths of their absorbance peaks were determined in a previous study [14]. The maximum absorption bands were 590 nm for RB-19, 600 nm for RB-5 and 490 nm for AR-AC. Absorbance-concentration calibration curves (Figure 1) were measured at these wavelengths for dye solutions within a concentration range of 0 to 150 ppm. All dye solutions contained a sodium hydroxide concentration of 0.33 M.
The calibration curves for AR-AC and RB-5 show lineal relationships. In case of RB-19 the absorbance-concentration relationship begins to be non-linear at concentrations over 75 ppm, consequently the traced graph was used to determinate the dye concentration.
• Experimental runs
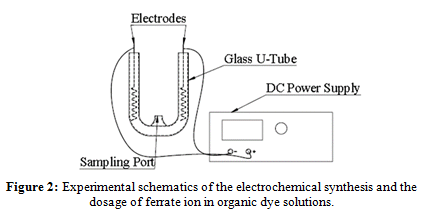
The electrochemical synthesis was carried out using a low voltage AC/DC power supply model SF-9584 Pasco Scientific. As presented in Figure 2, the reactor used was a glass U-Tube with a capacity of 180 mL. One electrode was placed in each extreme of the tube. The electrodes were made by two components: commercial non-steeled iron wire and steel wool. The wire had a length of 30 cm, of which 18 cm were coiled to hold the steel wool to the electrode. The steel wool was then wrapped to the wire coil, the overall weight of each electrode was of 9.3 g (5.5 g from the wire and 3.8 g from the steel wool). A DC power supply was used during the treatment for 2 hours at 15 V, 18 V and 20 V. The organic dye solutions were prepared in NaOH (commercial quality) solutions with molarities of 0.44 M and 0.33 M. In each trial, a total volume of 160 mL of sample was introduced into the reactor.
For the kinetics analysis, a 10 mL glass syringe was used for sampling. 4 mL samples were collected from the sampling port every 15 minutes for RB-5 and AR-AC dyes and every 10 minutes during the first hour treatment for the RB-19 dye.
3. RESULTS AND DISCUSSION
3.1. Characterization of organic dyes in sodium hydroxide solutions.
We studied the UV-vis spectra of the three dyes at different concentrations of NaOH solutions varying from 0 to 0.44 M. The dye concentration was kept constant at 150 ppm.
• Reactive Blue 19
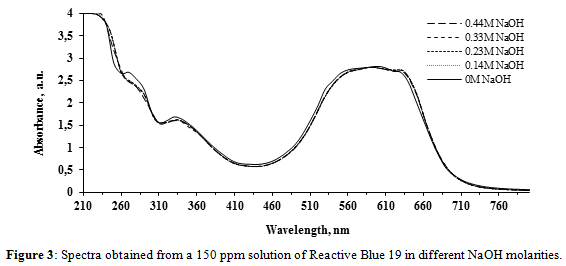
Figure 3 shows the spectra for RB-19. The absorbance values and the wavelengths corresponding to the absorbance peaks do not vary significantly in the range of 300 to 750 nm, showing that the NaOH molarities do not cause important chromic shifts. Nevertheless, significant hypochromic and hyperchromic effects are observed in the 240 – 300 interval, especially at 276 nm
• Allura Red AC
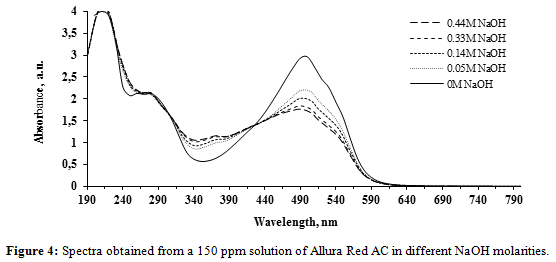
The NaOH concentrations effects on the spectra are substantially different for AR-AC, as shown in Figure 4. As the NaOH concentration increases (pH increase), significant hypochromic effects are observed in the range of 425 – 600 nm, with maximum effects at 495 nm where the absorbance peaks occur. Also a slight hypsochromic shift of this absorption band occurs. All these results are consistent with those reported by Bevziuk et al. [33]. An isobestic point appears at 425 nm and the absorbance intensities increase in the ranges of 305-420 and 235-265 nm, showing important hypercromic effects.
• Reactive Black 5
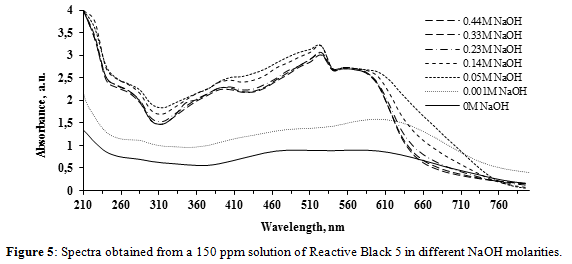
Figure 5 shows the effect of NaOH concentration in RB-5 dye solutions. When the NaOH molarity increased from 0 to 0.05 M, a strong hypercromic effect is observed over the whole wavelength range. Moreover, two absorbance peaks appear at 520 nm and 555 nm and the 600 nm peaks shift to the left to 555 nm, showing a strong hypsocromic effect of the solvent.
When the NaOH molarity further increases from 0.05 M to 0.44 M, the 555 nm peaks present no shifts, but the 520 peak diminishes and a new peak appears at 400 nm from NaOH 0.14 M. Furthermore, the absorbance response values decrease in wavelengths lower than 530 nm and greater than 580 nm.
3.2. Electrochemical oxidation by ferrate using commercial steel wool electrodes
Once the solvent effects on the behavior of RB-19, RB-5 and AR-AC dyes spectra were assessed, we studied the electrochemical oxidation by ferrate (VI) using commercial steel wool electrodes. Specifically we focused on the effects of current voltage and NaOH concentration, to determine optimal conditions for the simultaneous electrochemical synthesis and oxidation. Then we studied the kinetics of decolorization kinetics and the behavior of dye spectra, under the optimal conditions.
3.2.1. Effect of voltage and NaOH concentration
We studied the effects of voltage and NaOH concentrations on the color removal efficiency, after 2 h of reaction. Final pH and temperature values were also measured.
• Allura Red AC
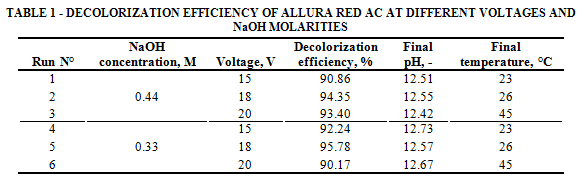
The results observed in Table 1 show that there is not a significant change in pH values during the treatment (12.57 average). As average, the decolorization efficiency is slightly higher using 0.44 M compared with 0.33 M of NaOH. These results match with those of previous studies focused on azo dye Orange II and the effects of initial pH in color degradation[22]. Even if a good current density is primordial for an optimal ferrate production[32], a too high voltage promotes the over production of hydrogen lowering the contact area between the electrode and the solution, as commented by Bouzek et al.[34]. The overheating of the solution represents also an issue when high voltages are applied (in our case 42-45°C at 20 V), decreasing the ferrate production as observed by Ding et al.[35]. Therefore, 18 V and 0.33 M NaOH are the optimal conditions for the color degradation, 96%.
• Reactive Blue 19
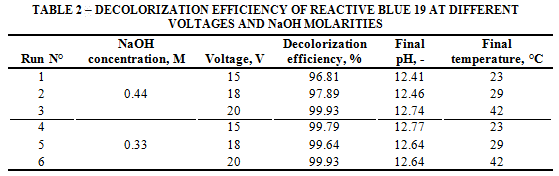
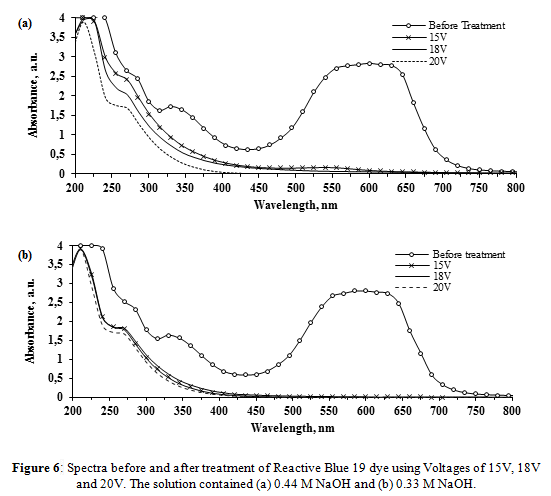
Final pH values of the solution are similar to those obtained with AR-AC. Regardless of the same temperature increase as observed in previous tests, the values at Table 2 show that voltage increased the color removal increased when the NaOH concentration was kept at 0.44 M. At 0.33 M, higher color removal efficiencies occurred, reaching values over 99.6%.
The spectra of the treated RB-19 solution (Figure 6) shows that the peaks in the visible region have disappeared. In the 210 - 400 nm interval, spectrums obtained with 0.44 M NaOH show a gradual intensity decrease, as the voltage increased. As it was previously commented, with a higher current density a higher production of ferrate and a higher oxidation of the dye components can be achieved. At 20 V, the results obtained with 0.33 M and 0.44 M are very similar. With 15 V and 18 V treatment there is a substantial difference in this UV region, with greater degradation shown with 0.33 M. As before, the best results were obtained with 0.33 M NaOH and 18 V. The resultant spectrum is comparable to the ones obtained with 20 V, with the exception that lower energy consumption and lower NaOH were used for the treatment.
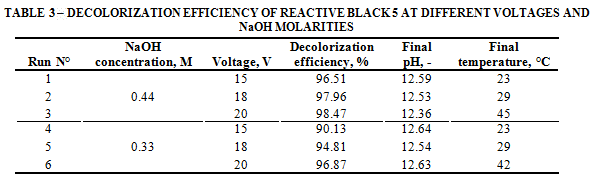
• Reactive Black 5
The values shown in Table 3 indicate that RB-5 has the same behavior as RB-19, as color degradation efficiencies increase when voltage increases. In this case, it was observed that at a higher NaOH concentration a better color removal was achieved, reaching a 98.5% removal at 20 V and NaOH 0.44 M. The final temperature increased in all the cases, as it was observed during AR-AC and RB-19 runs.
3.2.2. Color degradation kinetics
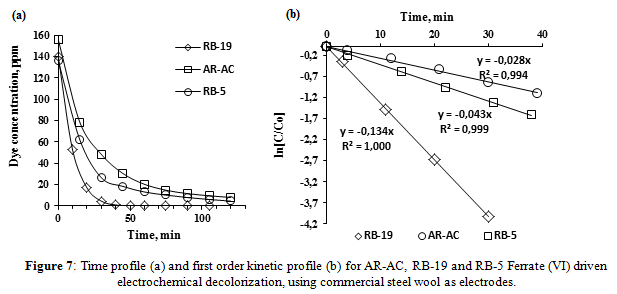
For the kinetic study, the simultaneous ferrate synthesis and dye oxidation was made using 150 ppm dye solutions in a 0.33 M sodium hydroxide matrix. 18 V were used for the treatment during 120 min. Time course concentration profiles of the three dyes are depicted in Figure 7(a). It may be noted that the extent of decolorization of the three dyes at the end of 120 min was higher than 94%. The corresponding first order kinetic plots shown in Figure 7(b) indicate that the initial rate of decolorization was much faster for RB-19 the anthraquinone type dye than for the RB-5 and AR-AC azo dyes.
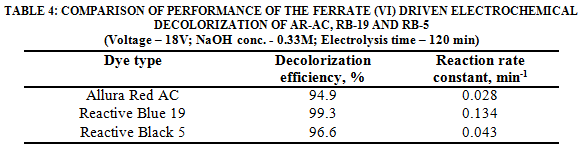
Table 4 summarizes the results of the color degradation using commercial steel wool as electrodes in the ferrate (VI) driven electrochemical oxidation.
3.2.3. Analysis of dye spectra
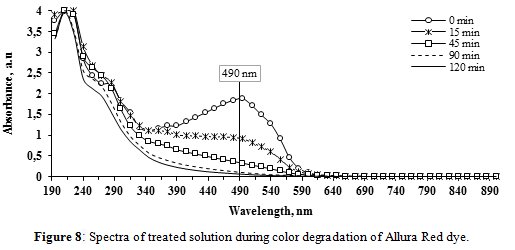
• Allura Red
The results of the spectra every 15 minutes are shown on Figure 8. The total color degradation was 94.9%, half of it occurring during the first 15 minutes of treatment. Before the beginning of the electrolysis, the UV-Vis spectrum of the dye-NaOH solution exhibited three peaks, one in the visible region (495 nm) and the other two in the UV region (210, 285 nm ). It was observed that the pick at 490 nm decreased consistently with time, showing an almost complete cleavage of the azo chromophores. In the range of 230-290 nm no new peaks emerged, but there was a slight increase of absorbance values up to 45 min, probably due to the formation and accumulation of less conjugated, partially oxidized intermediates, as commented by Popli & Patel[11]. Afterwards, these compounds were partially decomposed. These observations indicate that the main mechanism of decolorization was the oxidation of AR-AC.
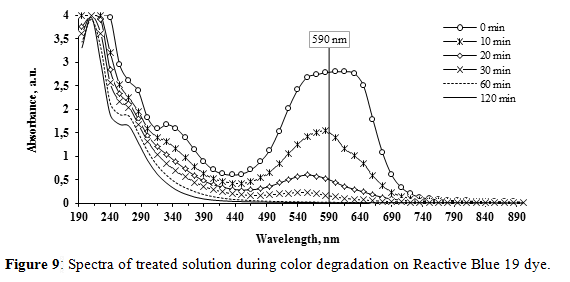
• Reactive Blue 19
The results of the spectra, measured every 10 minutes during the first hour and every 15 minutes during the second are shown in Figure 9. The total color removal was 99.3% with a 47% of it occurring during the first 10 minutes of treatment, showing a higher color degradation rate than in the case of AR-AC. A hypsochromic effect can be observed clearly after 20 minutes of treatment, as the main peak shifts from 590 nm to 555 nm. The solution color changed from blue to violet during the first minutes. During the last minutes of treatment a yellowish color developed.
Additionally the peak at 330 nm disappears also at the same time. A new peak seems to be formed around 280 nm as the treatment develops. A synchronic reduction of absorbance peaks occurred in the whole wavelength interval, especially in the visible range, indicating a complete cleavage of the quinoid chromophores and a partial oxidation of aromatic rings (peaks at 330 nm), without any formation or accumulation of intermediates. Again, these observations indicate that the main mechanism responsible for decolorization was the oxidation of RB-19.
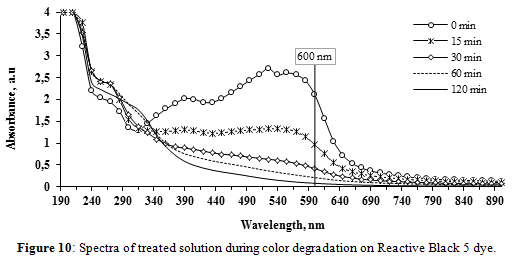
• Reactive Black 5
The results of the spectra were collected every 15 minutes during the treatment (Figure 10). During the first 15 min, the RB-5 color removal was around 50%, and a 97% was achieved in 120 min, showing a similar color degradation rate as AR-AC and slower rate compared with RB-19.
Before the beginning of the electrolysis, the UV-Vis spectrum of the dye-NaOH solution exhibited two peaks in the visible region, (555 and 525 nm) and one in the UV region (390 nm). The peaks at 555 and 525 nm decreased consistently with time, showing an almost complete cleavage of the azo chromophores. Nevertheless, up to 60 min, UV absorption in 200-320 nm range slightly increased probably due to formation and accumulation of less conjugated intermediates, as commented by Popli & Patel[11] who treated RB-5 by electro-oxidation using graphite anodes and NaCl as electrolyte. After 60 min, these intermediates are partially oxidized remaining in the final solution. Again, the oxidation of RB-5 was the predominant mechanism responsible for color removal.
4. CONCLUSIONS
• Solvent effects
Allura Red AC solutions are strongly affected by NaOH concentrations, presenting significant hypochromic effects in the visible region and important hyperchromic effects in the UV range, when NaOH molarities vary from 0 to 0.44 M. Reactive Black 5 water solutions are also affected by NaOH molarities from 0 to 0.44 M, showing very strong hyperchromic effects and significant hypsochromic shifts of the maximum absorption band in the visible region. Reactive Blue 19 water solutions are not significantly affected by NaOH concentrations varying from 0 to 0.44 M.
• Process kinetics
At the optimal conditions of voltage and NaOH concentration (18 V and 0.33 M), the decolorization of Reactive Blue 19 (anthraquinone-type dye) is much more rapid than those of azo dyes Reactive Black 5 and Allura Red AC. The decolorization rates of the three dyes can be expressed by a first order reaction model. The reaction rates were 0.134 min-1for RB-19, 0.043 min-1 for RB-5 and 0.028 min-1 for AR-AC. Color removal efficiencies higher than 95% were achieved at 120 min.
• Analysis of spectra
The absorption peaks in the visible region for the three dyes disappeared completely in 120 min, indicating a complete cleavage of both azo and quinoid chromophores.
In the case of the anthraquinone type RB-19 no new absorption peaks occurred in the UV region, showing a partial oxidation of aromatic groups without the generation of intermediates. In case of both azo-type dyes RB-5 and AR-AC, formation and accumulation of intermediates followed by their partial oxidation may have occurred, as absorbance intensities increased in the UV region and new peaks appeared.
All these observations indicate that the predominant mechanism for decolorization was the oxidation of the three dyes.
We conclude that that the electrochemical oxidation by ferrate (VI), under low voltages and low NaOH concentrations, using commercial steel wool as electrodes is an efficient and cost-effective alternative for the decolorization of azo and anthraquinone type dyes. For future studies a COD analysis should be made in order to correlate the decolorization and the elimination of the organic load in the dye solutions.
5. REFERENCES
[1] A. Camacho, Lineamientos para el plan estratégico sectorial de gestión de aguas residuales en lavanderías de jeans de Cochabamba. Editado y en cooperación técnica con Swisscontact, pp.17, 2016.
[2] A. Zaconeta Piva and R. Escalera Vásquez, Desarrollo de un sistema de reciclaje de aguas residuales textiles coloreadas mediante la utilización de un fotoreactor solar, Investigación & Desarrollo, vol. 1, no. 10, pp. 36–47, 2010.
[3] S. Barışçı, O. Turkay, and A. Dimoglo, Review on Greywater Treatment and Dye Removal from Aqueous Solution by Ferrate (VI), in Ferrites and Ferrates: Chemistry and Applications in Sustainable Energy and Environmental Remediation, vol. 1238, American Chemical Society, 2016, pp. 14–349.
[4] H. A. Akdogan, M. C. Topuz, and A. A. Urhan, Studies on decolorization of reactive blue 19 textile dye by Coprinus plicatilis, J. Environ. Health Sci. Eng., vol. 12, no. 1, p. 49, 2014.
[5] F. P. Van Der Zee, Anaerobic azo dye reduction, Wageningen University, 2002. [ Links ]
[6] G. Ciobanu, S. Barna, and M. Harja, Kinetic and equilibrium studies on adsorption of Reactive Blue 19 dye from aqueous solutions by nanohydroxyapatite adsorbent, Arch. Environ. Prot., vol. 42, no. 2, Jan. 2016.
[7] Y. Cheng, M. Lu, C. Jiao, and H.-J. Liu, Preparation of stabilized nano zero-valent iron particles via a rheological phase reaction method and their use in dye decolourization, Environ. Technol., vol. 34, no. 4, pp. 445–451, Feb. 2013.
[8] B. Armaǧan, O. Özdemir, M. Turan, and M. Çelik, The removal of reactive azo dyes by natural and modified zeolites: Removal of reactive azo dyes by zeolites, J. Chem. Technol. Biotechnol., vol. 78, no. 7, pp. 725–732, Jul. 2003.
[9] D. Rajkumar, B. J. Song, and J. G. Kim, Electrochemical degradation of Reactive Blue 19 in chloride medium for the treatment of textile dyeing wastewater with identification of intermediate compounds, Dyes Pigments, vol. 72, no. 1, pp. 1–7, Jan. 2007.
[10] V. M. Vasconcelos et al., Electrochemical removal of Reactive Black 5 azo dye using non-commercial boron-doped diamond film anodes, Electrochimica Acta, vol. 178, pp. 484–493, Oct. 2015.
[11] S. A. Popli and U. D. Patel, Electrochemical decolorization of Reactive Black 5 in an undivided cell using Ti and graphite anodes: Effect of polypyrrol coating on anodes, J. Electrochem. Sci. Eng., vol. 5, no. 2, Aug. 2015.
[12] F. Esteves and E. Sousa, CI Reactive Black 5 degradation by advanced electrochemical oxidation process, AEOP, pp. 1–6, 2007.
[13] İ. A. Şengil and M. Özacar, The decolorization of C.I. Reactive Black 5 in aqueous solution by electrocoagulation using sacrificial iron electrodes, J. Hazard. Mater., vol. 161, no. 2–3, pp. 1369–1376, Jan. 2009.
[14] J.-M. Fanchiang and D.-H. Tseng, Degradation of anthraquinone dye C.I. Reactive Blue 19 in aqueous solution by ozonation, Chemosphere, vol. 77, no. 2, pp. 214–221, Sep. 2009.
[15] Q. Zheng, Y. Dai, and X. Han, Decolorization of azo dye C.I. Reactive Black 5 by ozonation in aqueous solution: influencing factors, degradation products, reaction pathway and toxicity assessment, Water Sci. Technol., vol. 73, no. 7, pp. 1500–1510, Apr. 2016.
[16] S. Meriç, D. Kaptan, and T. Ölmez, Color and COD removal from wastewater containing Reactive Black 5 using Fentons oxidation process, Chemosphere, vol. 54, no. 3, pp. 435–441, Jan. 2004.
[17] P. Bahmani, A. Maleki, E. Ghahramani, and A. Rashidi, Decolorization of the dye reactive black 5 using Fenton oxidation, African J. Biotechnol., vol. 12, no. 26, pp. 4115–4122, 2013.
[18] M. Qiu, J. Shou, P. Ren, and K. Jiang, A comparative study of the azo dye reactive black 5 degradation by UV / TiO2 and photo-fenton processes, J. Chem. Pharm. Res., vol. 6, no. 7, pp. 2046–2051, 2014.
[19] M. Siddique, R. Khan, A. F. Khan, and R. Farooq, Improved Photocatalytic Activity of TiO2 Coupling Ultrasound for Reactive Blue 19 Degradation, J. Chem. Soc. Pak., vol. 36, no. 1, pp. 37–43, 2014.
[20] J.-M. Hong, Y.-F. Xia, C.-C. Hsueh, and B.-Y. Chen, Kinetic study of Reactive Black 5 degradation by Fe 2+ /S 2 O 8 2− process via interactive model-based response surface methodology, Water Sci. Technol., vol. 76, no. 7, pp. 1754–1769, Oct. 2017.
[21] Y. Lee, M. Cho, J. Y. Kim, and J. Yoon, Chemistry of ferrate (Fe (VI)) in aqueous solution and its applications as a green chemical, Journal of Industrial and Engineering Chemistry-Seoul-, vol. 10, no. 1. pp. 161–171, 2004.
[22] G. Li, N. Wang, B. Liu, and X. Zhang, Decolorization of azo dye Orange II by ferrate(VI)–hypochlorite liquid mixture, potassium ferrate(VI) and potassium permanganate, Desalination, vol. 249, no. 3, pp. 936–941, Dec. 2009.
[23] G. R. Xu, Y. P. Zhang, and G. B. Li, Degradation of azo dye active brilliant red X-3B by composite ferrate solution, J. Hazard. Mater., vol. 161, no. 2–3, pp. 1299–1305, Jan. 2009.
[24] Q. Han et al., Effects of coexisting anions on decolorization of azo dye X-3B by ferrate(VI) and a comparative study between ferrate(VI) and potassium permanganate, Sep. Purif. Technol., vol. 108, pp. 74–82, Apr. 2013.
[25] S. Sahinkaya, Decolorization of reactive orange 16 via ferrate (VI) oxidation assisted by sonication, Turk. J. Chem., vol. 41, pp. 577–586, 2017.
[26] X. Dong, L. Wang, X. Zhang, L. Bai, X. Zhang, H. Ma, C. Ma, and M. Xue, Oxidative degradation of azo dye Reactive Red 2BF by potassium ferrate, Adv. Mater. Res., vol. 523, pp. 2617–2620, 2012.
[27] Y. Li and M. Li, Treatment of acidic red-dye wastewater by ferrate (VI) oxidation, J. Shenyang Jianzhu Univ. (Natural Sci.) vol. 27, pp. 737–740., 2011.
[28] G. R. Xu, Y. P. Zhang, and G. B. Li, Degradation of azo dye active brilliant red X-3B by composite ferrate solution, J. Hazard. Mater., vol. 161, no. 2–3, pp. 1299–1305, 2009.
[29] P. M. Pérez García, S. L. Ibáñez-Calero, and R. Escalera Vásquez, Degradation of synthetic organic dyes in solution by ferrate - hypochlorite or calcium hypochlorite, Investigación & Desarrollo, vol. 1, no. 17, pp. 43–53, 2017.
[30] O. Turkay, S. Barışçı, and A. Dimoglo, Kinetics and mechanism of methylene blue removal by electrosynthesized ferrate (VI), Sep. Sci. Technol., vol. 51, no. 11, pp. 1924–1931, Jul. 2016.
[31] M. Villanueva, A. Hernandez, J. M. Peralta-Hernandez, E. R. Bandalab, and M. A. Quiróz, In-situ Electrochemical Generation of Ferrate Ion [Fe(VI)] in Acidic Conditions: A Potential Wastewater Decontamination Process, ECS Transactions, pp. 411–416, 2008.
[32] S. Barışçı, F. Ulu, H. Särkkä, A. Dimoglo, and M. Sillanpää, Electrosynthesis of Ferrate (VI) ion Using High Purity Iron Electrodes: Optimization of Influencing Parameters on the Process and Investigating Its Stability, Int. J. Electrochem. Sci., vol. 9, p. 19, 2014.
[33] K. Bevziuk, A. Chebotarev, D. Snigur, Y. Bazel, M. Fizer, and V. Sidey, Spectrophotometric and theoretical studies of the protonation of Allura Red AC and Ponceau 4R, J. Mol. Struct., vol. 1144, pp. 216–224, Sep. 2017.
[34] K. Bouzek, I. Rouar, and M. A. Taylor, Influence of anode material on current yield during ferrate(VI) production by anodic iron dissolution Part II: Current efficiency during anodic dissolution of white cast iron to ferrate(VI) in concentrated alkali hydroxide solutions, J. Appl. Electrochem., vol. 26, no. 9, pp. 925–931, 1996.
[35] Z. Ding, C. Yang, and Q. Wu, The electrochemical generation of ferrate at porous magnetite electrode, Electrochimica Acta, vol. 49, no. 19, pp. 3155–3159, Aug. 2004.