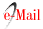Journal of the Selva Andina Animal Science
versão impressa ISSN 2311-3766versão On-line ISSN 2311-2581
J.Selva Andina Anim. Sci. vol.10 no.2 La Paz 2023 Epub 01-Out-2023
https://doi.org/10.36610/j.jsaas.2023.100200074
Tópicos Especiales
Characterization of water quality during freshwater culture of shrimp Litopenaeus vannamei in southern Ecuador
1Technical University of Machala. Faculty of Agricultural Sciences. Av. Panamericana km. 5 1/2 Vía a Pasaje. Tel. +593.2983362 - 2983365 - 2983363 - 2983364. Machala, Ecuador.
2BioMar Alimentsa Ecuador SA. Aparnor - Alborada 5, km 6. Av Isidro Ayora, Durán 090508. Tel: +593 4-371-1290. Tambo, Guayaquil, Ecuador.
3Independent Consultant. Santa Rosa and 11th Street North. Machala, Ecuador.
4Comercializadora Snapsi Cia Ltda. Julio Ramos Street N69-358 between B and C streets. Quito, Ecuador.
El presente trabajo caracteriza la calidad del agua durante el cultivo del camarón Litopenaeus vannamei en un sistema abierto, con el uso de agua dulce de pozo (0.3 ‰ salinidad). El agua subterránea contenía 0.3 ‰ de salinidad, 0.0115 mg L-1 de fosfato (P-PO4), 0.003 mg L-1 de nitrito (N-NO2), 0.029 mg L-1 de nitrato (N-NO3), < 0.010 mg L-1 de amonio N-NH4, 300 mg L-1 de dureza total y 210 mg L-1 de alcalinidad. El estudio comprende el análisis del proceso de aclimatación de post-larvas de L. vannamei de 20 días de edad y posterior desarrollo en un sistema abierto de cultivo. Durante la aclimatación la concentración de amonio se incrementó desde no detectada a 1.2 mg L-1 al segundo día, y luego a 1.5 mg L-1 al octavo día. La supervivencia de post-larvas al llegar al punto de agua dulce fue del 51 %. Durante 8 semanas de cultivo en los 2 estanques de tierra, la concentración de nutrientes fluctuó entre de 0.01 - 0.03 mg L-1 para nitrito, 0.02 - 0.03 para nitrato, 0.07 - 0.09 para amonio y 0.19 - 0.21 mg L-1 para fosfato. Los grupos fitoplanctónicos más representativos fueron las clorofitas, diatomeas, dinoflagelados y euglenoideos, con alrededor del 50.6, 25, 14 and 9.2 %, respectivamente en promedio para los dos estanques. El grupo de las cianofitas estuvo representado en el 1% de la comunidad de fitoplancton. Spirogyra sp., fue la especie más dominante. La concentración total de bacterias heterótrofas estuvo alrededor de 1100 UFC mL-1, mientras que Vibrio sp., Pseudomonas sp., y coliformes totales, alcanzaron una concentración promedio de 47, 72 UFC mL-1, y 59 UFC/mL-1 respectivamente. El crecimiento promedio semanal de L. vannamei fue de 0.9 g, la supervivencia de 45.9 %, y una producción alrededor de 1088 kg/ha. La naturaleza del agua de pozo en el Sur del Ecuador dispone de los nutrientes necesarios para el cultivo del camarón L vannamei a extrema baja salinidad.
Palabras clave: Aclimatación; acuacultura; agua freática; alcalinidad; baja salinidad; dureza; fitoplancton
The present work characterizes the water quality during the culture of shrimp Litopenaeus vannamei in an open system, using fresh well water (0.3 ‰ salinity). The water extracted from the subsoil contained 0.3 ‰ salinity, 0.0115 mg L-1 phosphate (P-PO4), 0.003 mg L-1 nitrite (N-NO2), 0.029 mg L-1 nitrate (N-NO3), < 0.010 mg L-1 ammonium N-NH4, 300 mg L-1 total hardness and 210 mg L-1 alkalinity. The study includes the analysis of the acclimation process of 20-day-old L. vannamei post-larvae and its further development in an open culture system. During acclimation, ammonium concentration increased from non-detect to 1.2 mg L-1 on the second day, and then to 1.5 mg L-1 on the eighth day. Survival of post-larvae upon reaching the freshwater point was 51 %. During 8 weeks of culture in the 2 earthen ponds, the nutrient concentration fluctuated between 0.01 - 0.03 mg L-1 for nitrite, 0.02 - 0.03 for nitrate, 0.07 - 0.09 for ammonium and 0.19 - 0.21 mg L-1 for phosphate. The most representative phytoplankton groups were chlorophyte, diatoms, dinoflagellates and euglenoids, with about 50.6, 25, 14 and 9.2 %, respectively in average for both ponds. The cyanophyte group was represented in the 1% of the phytoplankton community. Spirogyra sp. was the most dominant species. The total heterotrophic bacteria concentration was around 1100 CFU mL-1, while Vibrio sp., Pseudomonas sp. and total coliforms reached an average concentration of 47, 72 CFU mL-1, and 59 CFU/mL-1 respectively. The average weekly growth of L. vannamei was 0.9 g, survival was 45.9 %, and production was around 1088 kg/ha. The nature of the well water in southern Ecuador provides the necessary nutrients for the cultivation of L. vannamei shrimp at extremely low salinity.
Keywords: Aquaculture; acclimation; alkalinity; hardness; low salinity; ground water; phytoplankton
Introduction
Litopenaeus vannamei 1 (Boone, 1931), a crustacean commonly known as white shrimp (WS) naturally can be found in the Pacific coast, from Mexico to northern Peru, residing in marine and estuarine environments at salinities that range from 30 to less than 5 ‰. In Ecuador, the development of shrimp farming originated in mangrove areas2 using estuarine waters of the Gulf of Guayaquil. However, the strict control of mangrove deforestation2 and the appearance of diseases in shrimp were triggering factors for the search of new territories to continue the lucrative business of shrimp aquaculture. This action contributed to the increase in the cultivation area inland the continental shelf and the extraction of well water for shrimp farming. Under these conditions, the success of WS cultivation has been dependent on the physiological capacity of L. vannamei to adapt to low-salinity3. Consequently, the development of WS under culture conditions depends on its physiological capability to adapt to the physicochemical characteristics of the environment and the natural productivity that includes various phytoplankton groups.
Chemically, seawater contains chlorine, sodium, calcium, magnesium, potassium, bicarbonate, and sulfates, among the main elements that support the adaptation of aquatic species living in euryhaline environments. Due to its euryhaline condition, L. vannamei can adapt to various concentrations of salts, but due to limits of tolerance, high mortalities occur at less than 1 ‰ salinity4,5. Although it has not been documented yet, it is known that, in Ecuador, shrimp farming has expanded to freshwater culture systems with salt concentrations less than 0.5 ‰.
The expansion of the cultivation area in low-salinity environments requires technical and scientific data to understand the dynamics of shrimp production systems under low-salinity conditions6. Previous research pertaining to shrimp farming in low-salinity systems highlights differences in the ionic composition of groundwater from different regions (Alabama, USA, Thailand, Ecuador). In shrimp aquaculture systems, the low concentration of essential ions is amended by adding mineral salts to the culture environment7. As traditionally observed in Ecuador, in Thailand marine shrimp farming uses water sources located in lower river basins with salinities ranging from 2 to 5 ‰8,9 where the mixture with seawater allows the presence of essential minerals. However, in both environments estuarine and fresh water, inadequate proportions of calcium and magnesium can cause alterations in the health of the WS. Hence, the presence of anions (bicarbonate, carbonate, sulfates, chlorides) tested by the alkalinity of the water, and levels of hardness referred to the presence of ions (calcium, manganese and sodium) are critical be- cause mineral salts are vital for the maintenance of the WS8,10. Bicarbonate, an anion that forms alkalinity, is important due to its buffer capacity in water and its effect on pH changes, which in turn regulate the availability of essential ions in the aquatic environment.
The use of well water with salinities of 5 to 15 ‰ for shrimp farming inland or far from intertidal coastal zones has generated controversy due to environmental threats8,9,11, especially for the impact of water discharges containing salts that may affect agricultural crops. Extensive information has been documented on culture systems with low-salinity water, however, comprehensive information about the water quality for shrimp culture (SC) subjected to extremely low-salinity environments close to the point of fresh water, is limited. Thus, the opportunity to know the tolerance levels of L vannamei raised in fresh water or with minimal concentration of salts is essential for sustainable aquaculture. The inland shrimp farming using freshwater promotes the reuse of aquaculture wastewater for irrigation of agricultural crops or hydroponics12,13 and consequently reduce environmental impacts. Therefore, the objective of this research work was to analyze the adaptation capacity of shrimp to fresh water whose salt concentration was 0.3 ‰, and to characterize the water quality during the crustacean development in open culture systems.
Materials and methods
Study site. The study was performed in the Academic Unit of Agricultural Sciences, of the Technical University of Machala (UTM), Province of El Oro, Ecuador. The acclimation, and subsequent development of L vannamei was carried out under open culture systems and with the use of underground water at 0.3 ‰ salinity. The infrastructure consisted of one laboratory for the water and soil analysis and availability of 2 earthen ponds of 500 m2. The water supply consisted of a wellpoint system located close to the ponds. According to data of the local meteorological station, the site environmental conditions showed an average precipitation of 0.31 mm per day, relative humidity of 73 %, and temperature of around 25o C, typical characteristic of the dry season.
Table 1 Salinity variation according to the acclimation time of post-larvae L vannamei
| Acclimatization/observation days | Salinity 6:00 AM | Salinity 12:00 AM | Decreased salinity |
|---|---|---|---|
| 1 (PL-25) | 32 | 22 | 10 |
| 2 (PL-26) | 22 | 14 | 8 |
| 3 (PL-27) | 14 | 9 | 5 |
| 4 (PL-28) | 9 | 7 | 2 |
| 5 (PL-29) | 7 | 5 | 2 |
| 6 (PL-30) | 5 | 4 | 1 |
| 7 (PL-31) | 4 | 3 | 1 |
| 8 (PL-32) | 3 | 2 | 1 |
| 9 (PL-33) | 2 | 1 | 1 |
| 10 (PL-34) | 1 | .3 | .7 |
Acclimation of PL to low-salinity. The post-larvae (PL-4days old) were obtained from a local laboratory located in Puerto Bolívar, El Oro Province, Ecuador. The PL were transferred to the Aquaculture Program for the acclimation process from 32 ‰ to 0.3 ‰ salinity. A total of 75000 20-day-old PL were placed in three 500 L-1 tanks containing seawater of 30 ppm salinity, reaching a density of 50 PL L-1. The water temperature during the acclimation period varied around 26.4 ± 1.2o C. Every day, at the morning hours, the PL were gradually subjected to a reduction in salinity with the use of fresh water (from a well) until reaching the desired minimum concentration, followed with a subsequent observation and maintenance period during the afternoon and night time3 Table 1.
At the end of acclimation to 0.3 ‰ salinity, the PL were displaced in a volume of 1500 L, and held at a density of 15 PL L-1 for one week for observation prior to stocking in the earthen ponds.
Water quality analysis. During the entire culture cycle, 1 L water sample was taken for physicochemical, microbiological and phytoplankton analysis. Dissolved oxygen was measured twice (morning and afternoon) using a YSI model 55/DO portable oxygen meter. The pH was measured using the Hatch EC10 pH-Meter. Salinity was determined by the chloride titration method. Alkalinity was determined by the acidification and titration method, and total hardness by the EDTA12 method.
For the determination of nutrients, the water samples were taken in a separate container, and filtered through a 0.45 µm Whatman GF-C filter for further analysis following standard methods. After drying the filters, the weight was determined using a Denver Instrument analytical balance (model X-100). Nutrients were determined by spectrophotometry using the Hach DR 4000U UV/Visible Spectrophotometer. Ammonium (N-NH4) was determined through the indophenol12 reaction. Nitrite (N-NO2) by diazotization process13. Nitrate (N-NO3) by the reducing nitrate to nitrite using the cadmium reduction method12. The concentration of phosphates (P-PO4) was determined through the phosphomolybdate reaction and colorimetry14.
The qualitative analysis of phytoplankton was carried out using a Nikon Optiphot microscope. The phytoplankton species were identified with the support of reference manuals. The quantitative analysis of phytoplankton was carried out with the use of the Neubauer chamber. Additionally, a subsample was taken from the same water sample to estimate the number of heterotrophic bacteria by plate counting using Soybean-Casein Digest Agar Medium (TSA) (Difco, United States) and incubation at room temperature (28o C) for 48 h. The number of total coliforms was estimated using the MacConkey Agar. To estimate the number of Vibrio sp., the Thiosulfate-Citrate-Bile Salts-Sucrose Agar (Roth, Karlsruhe, Germany) was used, and for Pseudomonas sp., Cetrimide Agar, with incubation at 35º C for 24 h.
Shrimp culture in water with 0.3 ‰ salinity. SC was conducted in 500 m2 earthen ponds. After soil preparation, a pond bottom sample was taken to analyze the soil texture. The ponds received fresh well water 0.3 ‰ salinity. During the first week, 100 L of pure marine yeast with a concentration of 1 x 109 cell mL-1 was applied daily in each pond. On the fifth day of pond-water preparation, PL of 0.004 g weight were stocked at a density around 30 organisms m2, resulting in an average initial biomass of 0.6 kg in each pond. The maximum water level in the ponds was 80 cm.
During the culture period, every week 50 shrimp- were captured, from which 40 individuals were weighed, and 10 individuals were subjected to the analysis of phytoplankton microflora in the gut (intestine). The presence of phytoplankton microflora in the gut of the shrimp were evaluated weekly to confirm the preference of food. The shrimp were weighed on an Ohaus 400±0.1 g scale. The average weekly weight (PPS) was calculated by determining the total weight of the organisms (TP) and divided by the number of organisms (n).
PPS = PT/n
The weekly weight gain (IPS), was obtained by subtracting the PPS value of each week (PPS2) from the value of the previous week (PPS1): IPS= PPS2 (g) - PPS1 (g)
Based on the estimated biomass, artificial food with a protein composition of 27 % was provided manually in 2 daily rations.
The final survival rate (%S) was determined dividing the population that was stocked at the beginning (Pi) and the final population (Pf) at the end of the SC period after harvest.
%S = Pfx100/Pi
The feed conversion index was estimated as the ratio of the amount of feed used during cultivation and the final biomass.
Analysis of data. The data obtained were organized and analyzed descriptively in Excel spreadsheets, determining the average and standard deviation of the variables studied.
Results
The water source characteristics used for acclimation to low-salinity and further grow-out revealed 0.3 ‰ salinity, 0.0115 mg L-1 of phosphate, 0.003 mg L-1 of nitrite, 0.029 mg L-1 of nitrate, <0.010 mg L-1 of ammonium, 300 mg L-1 of total hardness and 210 mg L-1 of alkalinity. The soil texture of the ponds was 54.22 % silt, 38.9 % sand and 6.88 % clay.
Low-salinity acclimation. The concentration of ammonium in the water during the acclimation period fluctuated between 0.6 to 1.5 mg L-1, the pH fluctuated between 7.6 to 8.3, and temperature around 26o C. The final salinity, at the point of freshwater (0.3 ‰) was obtained at the age of PL-34-35 days of age. At the beginning of the acclimation period, the ammonium concentration reached 0.8 mg L-1. Later, halfway through the acclimation period (day 6), the ammonium concentration doubled in relation to the initial value, arriving to an average concentration of 1.2 mg L-1 (Figure 1). On the eighth day of acclimation, ammonium values were found at levels higher than 1.5 mg L-1, reducing slightly the next day, but increasing again on day 11. At the highest-level of ammonium PL showed abnormal behavior. As indicated, the pH of the water gradually increased during the acclimation from 7.6 to 8.1 and the temperature fluctuated around 26o C.
Figure 1 Variations of ammonium concentration in water during 11 days of acclimation of L. vannamei PL.
At the end of the acclimation, when the organisms were distributed in a volume of 1500 L of water, and with yeast addition, the ammonium concentration was reduced to a range of 0.35-0.8 mg L-1. This action helped to control the mortality observed at the end of the acclimation at 0.3 ‰.
Water quality characterization during grow-out. During the 60 days of SC, the physicochemical and microbiological parameters of the water were determined weekly in the two ponds (Table 2). The average concentrations of nitrite were 0.01 - 0.03 mg L-1, nitrate 0.02 - 0.03, ammonium 0.07 - 0.09 and phosphate 0.19 - 0.21 mg L-1 in the ponds 1 and 2 correspondingly. The inorganic nitrogen concentration in the water was lower than the phosphate concentration in both ponds. The average concentration of phosphate in the pond water was 20 times higher than the concentration found in the inlet water. The average concentration of nitrite in the pond water was 6.5 times higher than that of the inlet. The nitrate concentration in the pond water was close to that detected in the inlet. The ammonium concentration was around 0.08 mg L-1, increasing 20 times higher than the concentration detected in the inlet water.
Table 2 The average (± SD) of water quality variables in two experimental ponds during shrimp culture at 0.3 ‰ salinity
| Parameter | Pond 1 (P1) | Pond 2 (P2) |
|---|---|---|
| Phisicochemical | ||
| Alkalinity (mg L-1) | 239.75 ± 35.88 | 244.00 ± 25.06 |
| Total hardness (mg L-1) | 332.63 ± 114.34 | 351.88 ± 134.60 |
| Phosphates (P-PO4 mg L-1) | .19 ± .09 | .21 ± .18 |
| Nitrite (N-NO2 mg L1) | .01 ± .01 | .03 ± .04 |
| Nitrate (N-NO3 mg L-1) | .02 ± .02 | .03 ± .04 |
| Ammonium (N-NH4 mg L-1) | .07 ± .12 | .09 ± .14 |
| Microbiological | ||
| Vibrio (UFC mL-1) | 45.31 ± 41.10 | 48.00 ± 63.49 |
| Pseudomonas (UFC mL-1) | 71.62 ± 119.03 | 74.23 ± 128.89 |
| Coliformes (UFC mL-1) | 58.54 ± 183.26 | 61.00 ± 192.28 |
| Heterotrophic bacteria (UFC mL-1) | 988.83 ± 464.84 | 1218.83 ± 791.64 |
The daily variations of dissolved oxygen in the pond water fluctuated around 3.1±1.5 mg L-1. At the sixth week, the dissolved oxygen increased to 7 mg L-1 but within the same period, the oxygen decreased below 2 mg L-1. The survival rate in pond 2 was 9 % lower than that measured in pond 1, driving to a greater food consumption in pond 2.
In both ponds the microbial groups Vibrio sp., and Pseudomonas sp., were around 50 and 75 CFU mL-1 respectively (Table 2). The concentration of Pseudomonas sp., was 0.7 times higher than Vibrio sp., and the concentration of heterotrophic bacteria was around 103 CFU mL-1.
Phytoplankton characterization. The results of the qualitative analysis of primary productivity were similar for the 2 grow-out ponds. The concentration of total phytoplankton in the water in pond 1 reached levels around 56570 ± 4390 cell mL-1, while the concentration of total phytoplankton in pond 2 was around 25890 ± 3160 cell mL-1.
Although similar phytoplankton groups were observed in both ponds, the proportion of phytoplankton groups differed (Figure 2). The presence of chlorophytes was predominant in both ponds, occupying around 54,6 y 42.31 % of the total phytoplankton population for pond 1 and 2 respectively. Likewise, the diatoms were in second order of importance, reaching a proportion of 27.3 y 20%. The dinoflagellate group represented around 14 %. The presence of euglenoids was slightly different, reaching 11 and 19 % for pond 1 and 2 respectively. Ultimately, the cyanophycean group reached an average of 1 % of the phytoplankton population in the two ponds.
The gut microbiota of shrimps in pond 1 was represented by Spirogyra sp., (22 %) followed by the Diatoma sp. (21 %) Scenedesmus sp. (9.5 %), and in less proportion other types of microalgae were represented by Cocconeis, Chroococcus, Gymnodinium, Gonium, Merismopedia, Tabellaria, Pandorina, Thalassiosira plus dead zooplankton. The gut fullness ratio was around 7:1 for phytoplankton and artificial food respectively. Comparably, the shrimp gut content of the pond 2 presented the same type of microalgae but in a smaller number than pond 1. In both ponds the macroalgae Spirogyra sp., proliferated massively, although in pond 1 its appearance was significantly higher.
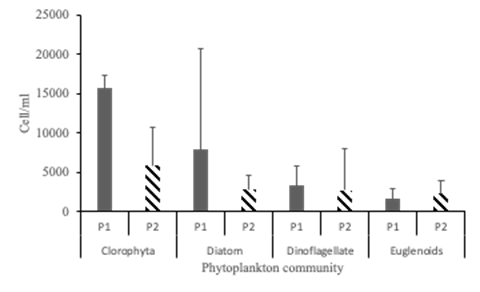
Figure 2 Distribution of predominant phytoplankton groups in shrimp culture with the use of 0.3 ‰ well water
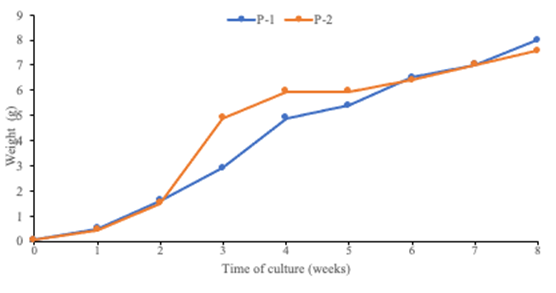
Performance of L vannamei at 0.3 ‰ salinity.Table 3 shows the production results of feed consumption, survival rate and feed conversion in the two ponds. The grow-out cycle began with an initial biomass of 0.55 and 0.65 kg and ended with a total of 57.3 and 51.5 kg of final biomass for ponds 1 and 2 respectively.
The shrimp L vannamei cultured under extremely low-salinity conditions, reached 0.92 g of weekly growth, and an average weight of 8 g in a period of 8 weeks. Halfway through the grow-out (45 days), a decrease in shrimp performance was recorded (Figure 3). The average production yield of the two ponds was 0.1 kg m-2 of total biomass. In pond 1 the records showed a feed consumption of 1.3 and in pond 2, the feed consumption increased around 30 %, which explains the higher feed conversion found in pond 2.
Discussion
Shrimp acclimation. L vannamei shrimp age is possibly one of the most critical points during acclimation to 0.3 ‰ salinity or at freshwater point. Other studies report significant differences in the survival rate of PL 10-days-old and 20-days-old when they approach levels close to the freshwater point, confirming that the tolerance of L vannamei to extremely low salinities depend on the age of the individuals15.
Table 3 Shrimp L. vannamei performance at 0.3 ‰ salinity
| Parameter | Pond 1 | Pond 2 |
|---|---|---|
| Initial shrimp population | 13820 | 16320 |
| Final shrimp population | 6917 | 6814 |
| Final weight (g) | 8.29 | 7.56 |
| Weekly growth rate | .97 | .88 |
| Final pond biomass (kg) | 57.3 | 51.5 |
| Feed consumption (kg) | 69.25 | 84.35 |
| Feed conversion | 1.3 | 1.7 |
| Survival (%) | 50.05 | 41.75 |
Indeed, it has also been suggested that acclimation to salinities close to the freshwater point requires more than 7 days of adaptation, resulting in high mortalities when acclimatize to extremely low-salinity16. Smaller PL are more susceptible to physiological alterations when exposed to stress conditions, showing low survival rate17.
Ammonium concentration is the most common toxic factor in low-salinity SC systems. In another study18, the exposure of 19-day-old L. vannamei showed that 50 % of the population died at N-NH3 concentration of 1.81 mg L-1. Another study with pre-juveniles of Penaeus schmitti, weighing 1.5 g, analyzed the susceptibility to ammonium at different salinities, and found that at 5 ‰ the concentration of 1.14 mg L-1 of N-NH3 was lethal for 50 % of the population19. The same study reported that ammonium causes physiological stress in organisms, leading to an increase in ammonium excretion and oxygen consumption. In this work, an increase in ammonium concentration was observed at the beginning of acclimation. Considering the pH and temperature of the water recorded in this work, the concentration of non-ionizable ammonium in the water was around 0.18 mg L-1 on the sixth day of acclimation and increased to 0.225 mg L-1 on the eleventh day, suggesting that ammonia could have influenced the survival of the PL during acclimation to 0.3 ‰ salinity. Observations in Ecuadorian hatcheries indicate that during the acclimation processes to salinities around 1 ‰, result in significant variations in the final survival response, which are not well understood, with some PL batches experiencing mortality rates as high as 75%.
Water quality characterization during grow-out in low-salinity. In the present work, the alkalinity and hardness of the water were the parameters on which this water quality study was focused. The concentration of nutrients, the primary productivity characterization, and the presence of pathogenic bacteria were analyzed.
The alkalinity and hardness of the water are fundamental parameters in shrimp farming20, suggesting a minimum of 100 mg L-1 of alkalinity21. In this study, with a salinity of 0.3 ‰, the alkalinity in the shrimp grow-out ponds was around 240 mg L-1 and the hardness values were about 340 mg L-1. This signifies that, the well water contained a significant proportion of calcium carbonate (Ca CO3). This could be due to natural characteristics of the geological conditions of the southern region of Ecuador. During cultivation, a slight reduction in alkalinity was observed, possibly due to the absorption of salts from the culture system or due to organism’s uptake. This aligns with other studies22 which suggests that alkalinity tends to decrease in L vannamei cultivated in low-salinity water. It has been reported that at an alkalinity of 40 mg L-1, shrimp growth can be delayed due to abnormal behavior during molting23. The suggested proportion of calcium and magnesium ions for L vannamei is around 60 and 40 %, respectively24. More specifically, Moura et al.25 reported optimal WS development in freshwater systems with 400 mg L-1 of calcium, 380 mg L-1 of potassium, 1350 mg L-1 of magnesium and 10500 mg L-1 of sodium. Araneda et al.26, in their study analyzing different densities of L vannamei cultivated in fresh water, showing alkalinity of 325 mg L-1 and hardness of 400 mg L-1. In addition, Jayasankar et al.16, when analyzing salinity and total hardness, found that the growth and survival of 20-day-old PL L. vannamei exposed to 30 ‰ salinity and 6600 mg L-1 hardness, was significantly higher than those organisms exposed to 5 ‰ and 1400 mg L-1 hardness, with a lower performance at 1.5 salinity and 450 mg L-1 hardness. The researchers confirmed that at 5 and 1.5 ‰ salinity the survival rate was 46 and 45 % respectively, comparable the results of the present work. This explains that the cultivation of L. vannamei in salinities up to 5 ‰ can achieve results equivalent to seawater by adjusting the alkalinity and hardness of the water to levels required for the WS. However, at extreme low salinities growth and survival decrease considerably.
In relation to nutrients dissolved in the water, the concentrations of phosphorus and inorganic nitrogen found in the two ponds occurred due to the dynamics of the system and the chemical flow from pond bottom to the water column, but nutrients did not enter from the inlet water. The concentrations of nitrogenous nutrients in the form of nitrites and ammonia were higher in the ponds than in the inlet water. The ammonium concentration in the grow-out pond did not represent a danger to the shrimps since it was between 0.07 to 0.08 mg L-1 for ponds 1 and 2 respectively. Previous studies using earthen ponds for SC indicate that phosphorus is generally not a limited nutrient22. Valenzuela-Quiñonez et al.5 analyzed four well water sources with salinities less than 1 ‰, during the cultivation of WS, reporting minimum and maximum concentrations for ammonium 0.26 to 0.31 mg L-1, nitrite 0.28 to 0.32 mg L-1, nitrate 0.73 to 0.77 mg L-1 and phosphate 1.5 to 1.7 mg L-1. In other studies, using the Hatch rapid analysis method, average values of nitrate of 6.7 mg L-1, total phosphorus 0.4 mg L-1 and reactive phosphorus 0.14 mg L-1 were reported, with the use of groundwater22, and a salinity of 2 ‰.
An additional aspect to remark about this work was the accumulation of the alga Spirogyra sp., producing a massive freshwater alga filament in the ponds. The presence of this macroalgae affected the monitoring and harvesting of the shrimp, since the organisms occupied these niches as a refuge and foraging. The presence of Spirogyra sp., detected in the gut analysis, corroborates the white shrimp's preference for this alga as a food source. Beyond the importance of primary productivity in the feeding behavior of shrimps, the presence of weed suggests analyzing the feasibility to create polyculture systems producing macroalgae, an aspect that requires further investigation.
Additionally, it is important to indicate that the well water was relatively clean and free of harmful microorganisms for L vannamei. The microbial groups of Vibrio sp., and Pseudomonas sp., in water emerged in low levels because of culture dynamics. In traditional culture systems, at the sea or when using estuarine water, shrimp farmers try to maintain the maximum limit of Vibrio sp., at around 102 CFU mL-1, although some reports show a range between 103 to 104 CFU mL-1. Therefore, it is considered that the low concentration of Vibrio sp., in shrimp farms is associated with the use of low-salinity groundwater; however, this cannot be generalized because low-salinity shrimp farms in Ecuador revealed concentration of Vibrio sp., over 104 CFU mL-1. The addition of fresh yeasts in the SC of this study could have contributed to enhance the quality of detritus as a nutritional complement for the shrimp and strengthening the presence of microorganisms antagonistic to Vibrio sp.29. To confirm this issue, the quantification of heterotrophic bacteria was around 103 CFU mL-1, while Pseudomonas sp., a bacterial group antagonistic to the presence of Vibrio sp.27,30, was higher. On the other hand, the appearance of coliforms in water explains the impact of sewage pollution within the area of the water source and experimental site.
Performance of L vannamei in water 0.3 ‰ salinity. The outcomes of L vannamei grow rate obtained in this work are comparable with other reports. In nature shrimp has a growth of 1.4 g per week4, and in seawater culture systems a weekly growth of 1.19 g per week. Likewise, Araneda et al.26, in Yucatán, Mexico, carried out laboratory tests cultivating the WS at 0 ‰ salinity, showing that the highest growth rate was obtained at densities of 90 shrimp m2, with an average of 0.38 g per week, and survival rate of 76 % in 210 days, concluding that in freshwater the survival decreases when the density of organisms is higher. These results are comparable with the present work, confirming that the greater the population of organisms in the aquatic environment, the availability of mineral salts to cope with the osmoregulation processes is more demanding, an issue that requires further investigation for better understanding.
Although massive mortality episodes, such as those commonly observed in traditional crops, were not observed, it is assumed that the population decline occurred progressively during shrimp cultivation. In the present study, it is important to highlight that during the culture period, no mineral supplement was administered to compensate the needs of salts. Hence, the WS cultivated in the present work only relied on the salts present in the water and the culture environment.
Additionally, since the pH of the soil in the ponds was neutral, the absorption of ions from the water is neglected31. But, in sandy soils conditions, there were losses due to infiltration, requiring the daily pumping of water up to 20 %. In extensive shrimp farming, water exchange rates vary around 2 - 7 % per day32, although 5 % water exchange per day has also been reported6. At the end of the growth-out period, the records showed that dissolved oxygen exceeded 100 % saturation, with drastic decreases during the night. The photosynthetic activity of microalgae can cause oxygen variations in the pond.
Considering the characteristics of open systems without mechanical aeration, the production obtained in the present work can be compared with outcomes of traditional SC systems, representing 1088 kg ha-1, a magnitude that can be increased with basic strategies to sustain the crustacean development. For example, transferring part of the population to adjacent ponds enhance the growth due to the reduction of population density, which has been reported as an alternative to enhance shrimp growth in two-phase systems, increasing the biomass and improving shrimp performance33.
Finally, this work offers a specific standpoint of the development and survival of L. vannamei with the use of underground water at 0.3 ‰ in an open culture system. The parameters of hardness, alkalinity, and ammonium are critical indicators for the acclimation process and subsequent development of shrimp in freshwater systems. The results have been compared and discussed with studies from other locations and laboratory tests, maintaining consistency. The water quality characterization during low-salinity SC enables better understanding of the pond dynamic managed under open systems, which contributes significantly to the optimization of SC. These results have allowed us to understand the productive potential of well water in Southern Ecuador. Hydrogeological studies to know the water table capacity are necessary for the sustainable management of underground water resources in context with the aquaculture operations.
REFERENCES
1. Boone L. Anomuran, Macruran crustacea from panama and canal zone [Internet]. New York: Bulletin of the American Museum of Natural History; 1931 [cited May 22, 2023]. 54 p. Retrieved from: https://decapoda.nhm.org/pdfs/13727/13727.pdf. [ Links ]
2. Jiménez Novillo JC, Carvajal Romero H, Vite Cevallos H. Analysis of the forecast of shrimp exports in Ecuador from the year 2019. Revista Metropolitana de Ciencias Aplicadas 2021;4(1):56-61. [ Links ]
3. Nunes AJP, Velásquez-López C. Low-salinity, inland shrimp culture in Brazil and Ecuador [Internet]. Global Seafood Alliance. 2023 [cited 3 May 2023]. Retrieved from: https://www.globalseafood.org/advocate/low-salinity-inland-shrimp-culture-in-brazil-and-ecuador/. [ Links ]
4. Valenzuela-Madrigal IE, Valenzuela-Quiñónez W, Esparza-Leal HM, Rodríguez-Quiroz G, Aragón-Noriega E. Effects of ionic composition on growth and survival of white shrimp Litopenaeus vannamei culture at low-salinity well water. Rev Biol Mar Oceanogr 2017;52(1):103-12. DOI: https://doi.org/10.4067/S0718-19572017000100008. [ Links ]
5. Valenzuela-Quiñónez W, Esparza-Leal HM, Nava-Pérez E, Rodríguez Quiroz G. El cultivo de camarón en agua de baja salinidad con alimento a base de harina de lombriz. Ra Ximhai 2012;8(3): 131-6. DOI: https://.doi.org/10.35197/rx.08.03.e2.2012.12.wv. [ Links ]
6. Hernández Gurrola JA. Caracterización de la calidad de agua en un sistema intensivo de cultivo de camarón blanco Litopenaeus vannamei, en condiciones de alta salinidad con recambio de agua limitado [tesis maestría]. [La Paz, Baja California Sur]: Centro de Investigaciones Biológicas del Noroeste, S.C; 2016 [citado 26 de mayo de 2023]. Recuperado a partir de: https://dspace.cibnor.mx:8080/handle/123456789/505. [ Links ]
7. Boyd CE, Thunjai T, Boonyaratpalin M. Dissolved salts in water for inland, low-salinity shrimp culture [Internet]. Global Seafood Alliance. 2023 [cited May 22, 2023]. Retrieved from: https://www.globalseafood.org/advocate/dissolved-salts-in-water-for-inland-low-salinity-shrimp-culture/#:~:text=Brine%20solutions%20of%20around%20200%2C000,use%20in%20inland%20shrimp%20culture. [ Links ]
8. Szuster BW, Flaherty M. Cumulative environmental effects of low salinity shrimp farming in Thailand. Impact Assess Proj Apprais 2002;20 (3):189-200. DOI: https://doi.org/10.3152/147154602781766672. [ Links ]
9. Biao X, Zhuhong D, Xiaorong W. Impact of the intensive shrimp farming on the water quality of the adjacent coastal creeks from Eastern China. Mar Pollut Bull 2004;48(5-6):543-53. DOI: https://doi.org/10.1016/j.marpolbul.2003.10.006. [ Links ]
10. Roy DA, Saoud IP, Boyd CA, Pine HJ, Boyd CE. Shrimp culture in inland low salinity waters. Rev Aquac 2010;2(4):191-208. DOI: https://doi.org/10.1111/j.1753-5131.2010.01036.x. [ Links ]
11. Flaherty M, Szuster B, Miller P. Low salinity inland shrimp farming in Thailand. Ambio 2000; 29(3):174-9. DOI: https://doi.org/10.1579/0044-7447-29.3.174. [ Links ]
12. McIntosh D, Fitzsimmons K. Characterization of effluent from an inland, low-salinity shrimp farm: what contribution could this water make if used for irrigation Aquac Eng 2003;27(2):147-56. DOI: https://doi.org/10.1016/S0144-8609(02)00054-7. [ Links ]
13. Miranda FR, Lima RN, Crisóstomo LA, Santana MGS. Reuse of inland low-salinity shrimp farm effluent for melon irrigation. Aquac Eng 2008; 39(1):1-5. DOI: https://doi.org/10.1016/j.aquaeng.2008.04.001. [ Links ]
14. Solorzano L. Métodos de análisis químicos utilizados en el curso latinoamericano de post-grado: "instrumentación y análisis químicos de agentes contaminantes en el mar" (1984). Boletín Científico y Técnico 1984; 7(1):x. [ Links ]
15. Strickland JDH, Parsons TR. A Practical Handbook of Seawater Analysis [Internet]. Ottawa: Fisheries Research Board of Canada; 310 p. DOI: https://doi.org/10.25607/OBP-1791. [ Links ]
16. Hansen HP, Koroleff F. Determination of nutrients. In: Grasshoff K, Kremling K, Ehrhardt M, editors. Methods of Seawater Analysis. Toronto: John Wiley & Sons, Inc; 1999. p. 159-228. DOI: https://doi.org/10.1002/9783527613984.ch1. [ Links ]
17. McGraw WJ, Davis DA, Teichert-Coddington D, Rouse DB. Acclimation of Litopenaeus vannamei postlarvae to low salinity: influence of age, salinity endpoint, and rate of salinity reduction. J World Aquac Soc 2002;33(1):78-84. DOI: https://doi.org/10.1111/j.1749-7345.2002.tb00481.x. [ Links ]
18. Jayasankar V, Safiah Jasmani S, Nomura T, Nohara S, Huong DTT, Wilder MN. Low salinity rearing of the pacific white shrimp Litopenaeus vannamei: acclimation, survival and growth of postlarvae and juveniles. Jpn Agric Res Q 2009; 43(4):345-50. DOI: https://doi.org/10.6090/jarq.43.345. [ Links ]
19. Balbi RJ, Velásquez CT, Maneiro. Aclimatación a baja salinidad de postlarvas del camarón marino Litopenaeus vannamei (Boone, 1931) provenientes de dos criaderos comerciales. Rev Biol Mar Oceanogr 2005;40(2):109-15. [ Links ]
20. Barajas FM, Villegas RS, Clark GP, García Mosqueda J, López Moreno B. Daily variation in short-term static toxicity of unionized amoníaco in Litopenaeus vannamei (Boone) postlarvae. Aquac Res 2006;37(14):1406-12. DOI: https://doi.org/10.1111/j.1365-2109.2006.01573.x. [ Links ]
21. Barbieri E. Acute toxicity of ammonia in white shrimp (Litopenaeus schmitti) (Burkenroad, 1936, Crustacea) at different salinity levels. Aquaculture 2010;306(1-4):329-33. DOI: https://doi.org/10.1016/j.aquaculture.2010.06.009. [ Links ]
22. Molina C, Espinoza M, Chuya N. Improving the osmoregulatory capacity of Pacific white shrimp grown in low salinity [Internet]. Global Seafood Alliance. 2019 [cited 3 May 2023]. Retrieved from: https://www.globalseafood.org/advocate/improving-the-osmoregulatory-capacity-of-pacific-white-shrimp-grown-in-low-salinity/. [ Links ]
23. Van Wyk P, Davis-Hodgkins M, Laramore R, Main KL, Mountain J, Scarpa J. Farming marine shrimp in recirculating freshwater systems [Internet]. Florida: Harbor Branch Oceanographic Institution; 1999 [cited Mar 22, 2023]. 34 p. Retrieved from: https://www.researchgate.net/publication/242621708_Farming_Marine_Shrimp_in_Recirculating_Fresh_Water_Systems. [ Links ]
24. Quimis Puga KL, Rodriguez Vera HS. Water quality in an intensive white shrimp culture system Penaeus vannamei in low salinity conditions [tesis licenciatura]. [Guayaquil]: Escuela Superior Politécnica del Litoral; 2019 [citado 26 de mayo de 2023]. Recuperado a partir de: https://www.dspace.espol.edu.ec/handle/123456789/51424. [ Links ]
25. Prapaiwong N, Boyd CE. Effects of major water quality variables on shrimp production in inland, low-salinity ponds in Alabama. J World Aquac Soc 2012;43(3):349-61. DOI: https://doi.org/10.1111/j.1749-7345.2012.00572.x. [ Links ]
26. Moura PSD, Wasielesky W Jr, Serra FDP, Braga A, Poersch L. Partial seawater inclusion to improve Litopenaeus vannamei performance in low salinity biofloc systems. Aquaculture 2021;531: 735905. DOI: https://doi.org/10.1016/j.aquaculture.2020.735905. [ Links ]
27. Araneda M, Pérez EP, Gasca-Leyva E. White shrimp Penaeus vannamei culture in freshwater at three densities: condition state based on length and weight. Aquaculture 2008; 283(1-4):13-8. [ Links ]
28. Liu X, Li Z, Cao Y, Wen G. Common species composition, quantity variation and dominant species of planktonic microalgae in low salinity culture ponds. South China Fisheries Science 2008;5(1):9-16. [ Links ]
29. Maicá PF, de Borba MR, Wasielesky Jr W. Effect of low salinity on microbial floc composition and performance of Litopenaeus vannamei (Boone) juveniles reared in a zero-water-exchange super-intensive system. Aquac Res 2012;43(3):361-70. DOI: https://doi.org/10.1111/j.1365-2109.201102838. [ Links ]
30. Solorzano-Reyes F, Velásquez-López PC. Eficiencia de absorción en postlarvas del camarón Litopenaeus vannamei alimentado con una dieta de levaduras marinas de marismas de manglares. Bol Invertir Mar Costo 2021;50(2):73-90. DOI: https://doi.org/10.25268/bimc.invemar.2021.50.2.1012. [ Links ]
31. Chythanya R, Karunasagar I, Karunasagar I. Inhibition of shrimp pathogenic vibrios by a marine Pseudomonas I-2 strain. Aquaculture 2002;208(1-2):1-10. DOI: https://doi.org/10.1016/S0044-8486(01)00714-1. [ Links ]
32. Palacios Serrano NO. Estudio de factibilidad para producir camarón de la especie Litopenaeus vannamei bajo un sistema de producción semi-intensivo en Ecuador [tesis licenciatura]. [Zamorano]: Escuela Agrícola Panamericana, Zamorano; 2016 [citado 16 de mayo de 2023]. Recuperado a partir de: https://bdigital.zamorano.edu/items/0d8e8ca3-32bb-4363-b734-d010cf6ed7ca. [ Links ]
33. Miranda I, Valles JL, Sánchez R, Álvarez Z. Cultivo del camarón marino Litopenaeus Vannamei (Boone, 1931) en agua dulce. Rev Cient (Maracaibo) 2010;20(4):339-46. [ Links ]
34. Castillo-Ochoa B, Velásquez-López PC. Manejo estacional de los sistemas de producción de camarón en el Ecuador [Internet]. Vol. 4, Sociedad & Tecnología. 2021; p. 447-61. DOI: https://doi.org/10.51247/st.v4i3.151. [ Links ]
Conflict of interest The authors of this study certify that there are no conflicts of interest regarding the research.
Acknowledgments This work was developed through the Water Quality Project P-BID-148 of the Aquaculture Program of the Faculty of Agricultural Sciences at the Technical University of Machala, and with the sponsorship of the Foundation for Science and Technology of Ecuador.
Ethical considerations The research had the approval of the Ethics Committee of the Association of Shrimp Producers of the Province of El Oro (Ecuador) and was conducted under ethical criteria of the Secretariate of Science and Technology, and the larvae producer company.
Contribution of the authors in the articleJuana Fulvia Solorzano Reyes, control and management of post-larvae during the acclimation process and control of shrimp feeding during culture in ponds. Patricia Migdalia Ochoa Pereira, analysis and quantification of bacteria and phytoplankton and assistance in the preparation of the original draft of the manuscript according to guidelines to be published. Galo Solano Motoche, chemical analysis of water during acclimation and cultivation, and manuscript review. Patricio Quizhpe Cordero, assistance in monitoring shrimp growth in pools. Roy Guillen Añazco, assistance in crop management and supervision of water quality in ponds, water exchanges. Patricio Colón Velásquez López, research conceptualization, supervision, analysis, and interpretation of water quality and managing the production system data, writing and editing of the manuscript, preparation of tables and figures, and literature review.
Limitations There were some logistical limitations during the shrimp culture in open systems, which in some way restricted the opportunity to replicate and continue the crop to a longer period.
Editor's Note: Journal of the Selva Andina Animal Science (JSAAS). All statements expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, editors and reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Received: March 01, 2023; Revised: May 01, 2023; Accepted: August 01, 2023










 texto em
texto em 
 uBio
uBio