Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista CON-CIENCIA
Print version ISSN 2310-0265
Rev.Cs.Farm. y Bioq vol.8 no.2 La Paz Nov. 2020
ARTÍCULOS ORIGINALES
Structural dynamics between the open and closed conformations of
the KATP channel in pancreatic cells
Dinámica estructural entre la conformación abierta y cerrada del
canal KATP en células pancreáticas
Ballon Paucara Wendy Guadalupe1![]() , Grados-Torrez Ricardo Enrique1*
, Grados-Torrez Ricardo Enrique1*![]()
1 Area de Farmacología, Instituto de Investigaciones Fármaco Bioquímicas Luis Enrique Terrazas Siles. Universidad Mayor
de San Andrés, Av. Saavedra 2224. La Paz, Bolivia.
*Author for correspondence: Wendy Guadalupe Ballon Paucara Licenciada en Bioquímica.
Unidad de Bioquímica Clínica, Área de Farmacología. Instituto de Investigaciones Fármaco Bioquímicas Luis Enrique
Terrazas Siles Facultad de Ciencias Farmacéuticas y Bioquímicas. Universidad Mayor de San Andrés.
E-mail: wen.ballon.wen@gmail.com
Principal author: Ricardo Enrique Grados Torrez (Author for correspondence).
PhD en Biotecnología, MSc. en Biotecnología Molecular, Lic. en Bioquímica. Área de Farmacología, Instituto de
Investigaciones Fármaco Bioquímicas (I.I.F.B.) Luis Enrique Terrazas Siles. Universidad Mayor de San Andrés,
Av. Saavedra 2224. La Paz, Bolivia.
E-mail: ric.grados@gmail.com Cel. 65192791
Fecha de recepción: 22 Septiembre 2020 Fecha de aceptación: 12 Octubre de 2020
Abstract
Introduction: The ATP-sensitive Potassium channel (KATP channel) regulates insulin production by pancreatic β cells. Glibenclamide (GBM) (antidiabetic drug) and ATP act as inhibitors of this channel, while ADP activates it. The KATP channel is an octamer consisting of 4 central Kir6.2 subunits that form the pore and 4 external regulation subunits SUR1.
Objective: To determine the structural dynamics between the open and closed conformations of the KATP channel in pancreatic cells.
Method: Comparative structural analysis of different crystallographic structures of the KATP channel of human pancreatic cells using Chimera v1.11.2.
Results: The Kir6.2 subunit has a PIP2 binding domain (activator), an Interfacial Helix (IFH) and an N-terminal domain (KNtp). On the other hand, the SUR1 subunit that contains the GBM binding site, has 2 Nucleotide Binding Domains (NBD1/2), an M5-Lh1 loop and a Lasso Motif formed by the interface between the Trans-membrane Domain 0 and Loop 0 (TMD0-L0). The results of the dynamic structural analysis using bioinformatics tools indicate that these regions participate actively in the conformational changes that lead to the closure (inhibition) or opening (activation) of this channel.
Conclusion: The study of the dynamics of activation and inhibition of the KATP channels is essential for the evaluation, discovery and/or design of new natural compounds, which like GBM, can promote insulin secretion to aid or improve the treatment of diabetic patients.
Keywords: KATP Channel, Structural Dynamics, Kir6.2, SUR1, Glibenclamide, ATP and ADP.
Resumen
Introducción: El canal de Potasio sensible a ATP (canal KATP) regula la producción de Insulina por células β pancreáticas. La Glibenclamida (GBM) (fármaco antidiabético) y el ATP actúan como inhibidores de este canal, mientras que el ADP lo activa. El canal KATP es un octámero constituido por 4 subunidades centrales Kir6.2 que forman el poro y 4 subunidades externas de regulación SUR1.
Objetivo: Determinar la dinámica estructural entre las conformaciones abierta y cerrada del canal KATP en células pancreáticas.
Método: Análisis estructural comparativo de diferentes estructuras cristalográficas del canal KATP de células pancreáticas humanas empleando el software Chimera v1.11.2.
Resultados: La subunidad Kir6.2 presenta un dominio de unión a PIP2 (activador), una Hélice Interfacial (IFH) y un dominio N-terminal (KNtp). Por otro lado, la subunidad SUR1 que contiene el sitio de unión a la GBM, tiene 2 Dominios de Unión a Nucleótidos (NBD1/2), un bucle M5-Lh1 y un Motivo de Lazo formado por la interface entre el Dominio Trans-membrana 0 y el Bucle 0 (TMD0-L0). Los resultados del análisis dinámico estructural mediante herramientas bioinformáticas, indican que estas regiones participan activamente en los cambios conformacionales que dan lugar al cierre (inhibición) o apertura (activación) de este canal.
Conclusión: El estudio de la dinámica de activación e inhibición de los canales KATP es imprescindible para la evaluación, descubrimiento y/o diseño de nuevos compuestos naturales, que como la GBM, puedan promover la secreción de Insulina para coadyuvar o mejorar el tratamiento de pacientes diabéticos.
Palabras clave: Canal KATP, Dinámica Estructural, Kir6.2, SUR1, Glibenclamida, ATP y ADP
INTRODUCTION
Insulin controls the concentration of Glucose in the blood. When this concentration is high, a group of pancreatic β cells release this hormone. In these cells, the amount of sugar in the blood modifies the ATP/ADP ratio and a membrane protein called ATP-sensitive Potassium channel (KATP channel) acts as a switch that activates or deactivates the production of Insulin. ATP and ADP control this switch, since both have opposite effects on this channel (ATP act as inhibitor and ADP as activator) (Aittoniemi et al., 2009), therefore, their action is coupled with the release of Insulin (Aguilar-Bryan et al., 2001; Ashcroft, 2005). However, it is not yet clear how KATP channels detect changes in the ATP/ADP ratio in these cells, and because ATP levels are usually high and constant, ATP is continuously deactivating these channels while it is still unclear how ADP activates those (Lee et al., 2017). On the other hand, Glibenclamide (GBM), sulfonylurea used in the conventional treatment of Type 2 Diabetes Mellitus (T2DM), also inhibits KATP channels promoting Insulin secretion. Understanding the conformational changes that occur in the KATP channel is important for the discovery of new natural compounds that promote Insulin secretion in patients with T2DM.
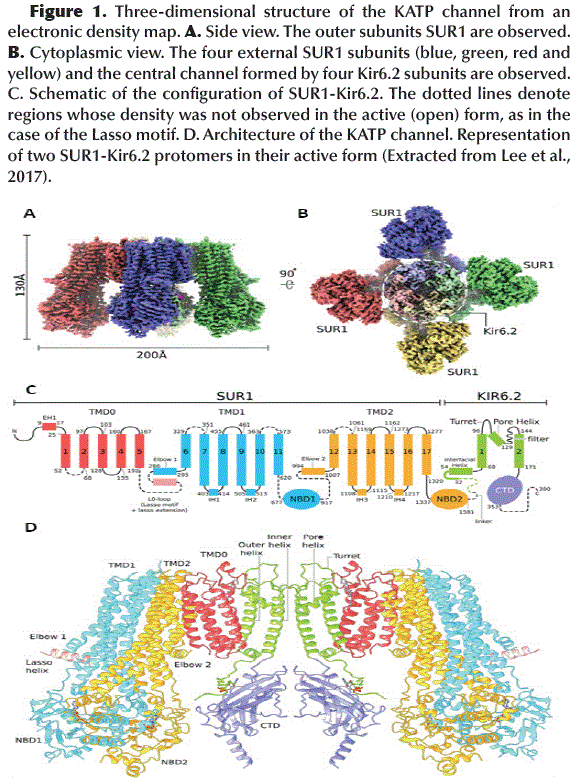
Structure of KATP channel
Functional KATP channels are membrane proteins that have a hetero-octameric structure composed of four Internal Rectification Subunits (Kir6.2) that close in depolarization maintaining the membrane potential and four Sulfonylurea Receptor Subunits (SUR1) members of the ABCC family (ATP Binding Cassette, subfamily C) (Lee et al., 2017) (Figure 1A and B), with a total of 80 trans-membrane helices (3 helices for each Kir6.2 subunit and 17 helices for each SUR1 subunit) (Figure 1C) and with a total molecular weight of about 880 kDa (Li et al., 2017; Martin et al., 2017b). The KATP channel pore is made up of Kir6.2 subunits that act as ATP sensors and their activity is regulated by PIP2. The SUR1 subunits mediate the activating effect of ADP-Mg2+ and determine the pharmacological profile of the channel (Wu et al., 2018). It should be noted that the KATP channels have different combinations of subunits and different pharmacological profiles in different tissues, but the specific KATP channels of pancreatic β cells are mainly composed of Kir6.2 and SUR1 subunits and are highly sensitive to anti diabetic drugs such as GBM (Ashcroft, 2005; Martin et al., 2019).
Kir6.2
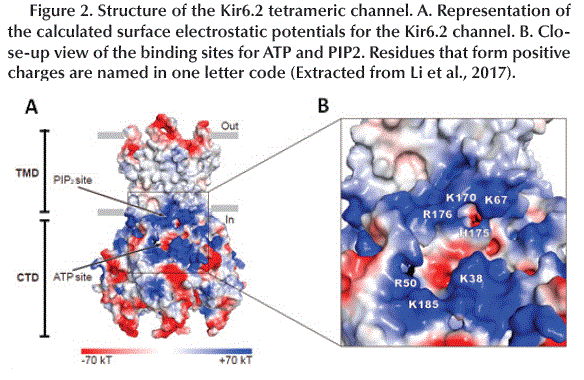
The tetrameric structure of Kir6.2 subunits forms the channel itself and has two-layer architecture, a Trans-Membrane Domain (TMD) and a Globular Cytosolic Domain (CTD) (Figure 2A). The TMD has three trans-membrane helices: the Pore Helix (PH), an Inner Helix (IH or M2) that connects to the CTD and one the Outer Helix (OH or M1) that connects to the cytoplasm with a small Interfacial Helix (IFH) which has a final extension in the form of a βA-IFH loop (Figure 1C and D), this loop is connected to the N-terminal region (KNtp) which is highly dynamic (Li et al., 2017).
The surface electrostatic potential shows two positively charged regions. The first consists of K67, K170, H175 and R176 which is known as the PIP2 binding site which is an activator of KATP channels (open the channel). The second region is formed by R50, K38 and K185 located near the cavity that accommodates the Adenosine group of ATP (ATP binding site) (Hansen et al., 2011) (Figure 2B).
SUR1
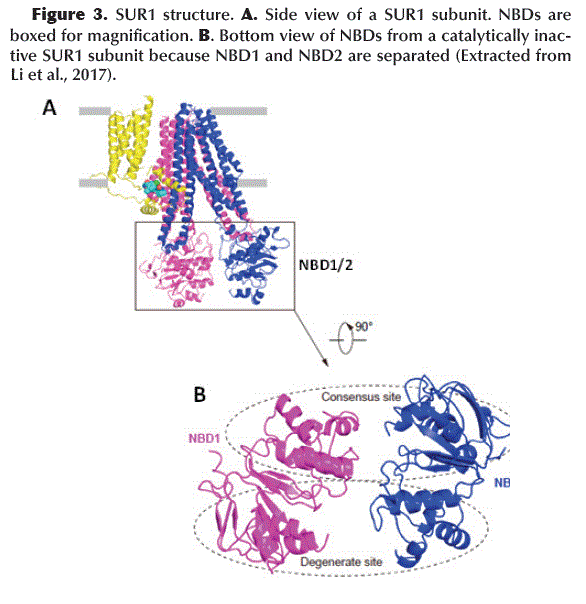
The Kir6.2 tetrameric channel is surrounded by four SUR1 subunits. Each divided into a TMD0 (with 5 trans-membrane helices: M1-5) with adjacent cytosolic loop L0 (Loop 0) and an ABC transporter-like domain TMD1-TMD2 (with 12 trans-membrane helices: TMD1 M6-11 and TMD2 M12-17) (Aittoniemi et al., 2009) (Figure 1C). SUR1 TMD1-TMD2 has an inward-facing conformation since the central cavity is completely accessible to the cytosolic face. The interface formed between TMD0-L0 and TMD1-TMD2 is called the Lasso Motif (Figure 1C and D). Inwardly, TMD1 and TMD2 possess a Nucleotide Binding Domain NBD1 and NBD2, respectively (Figure 3A), which form a catalytic site for ATP hydrolysis when both NBDs acquire a dimeric conformation (one close to the other) (Zhang and Chen, 2016). ABC-like domains have a characteristic motif (LSGGQ) and a Glutamate (E855). However, in SUR1 the NBDs are functionally asymmetric, since NBD1 presents an Aspartate instead of Glutamate (E855D) and NBD2 does not retain the characteristic ABC motif, therefore, one site is consensus and the other is degenerated (Vedovato et al., 2015) (Figure 3B).
METHOD
In this study, a comparative structural analysis of different crystallographic structures of the KATP channel of human pancreatic cells was performed, obtained from the Protein Data Bank database (PDB: http://www.rcsb.org/).
6baa: Crystallographic structure of KATP channel from pancreatic β cells bound to ATP and GBM, this structure was used for the analysis of the GBM binding site.
5ykf: Crystallographic structure of KATP channel from pancreatic β cells bound to ATPγS and GBM, this structure was used for the analysis of conformational changes in SUR1 and the NBD1 and NBD2 domains when the channel is in its closed conformation.
5ywc: Crystallographic structure of the KATP channel from pancreatic β cells bound to ADP-Mg2+, this structure was used for the analysis of conformational changes in SUR1 and the NBD1 and NBD2 domains when the channel is in its open conformation.
6pza: Crystallographic structure of the SUR1 subunit from pancreatic β cells bound to ATP and GBM, this structure was used for the analysis of the Lasso Motif (TMD0-L0) and the N-terminal domain of Kir6.2 (KNtp).
All the structures were analyzed, evaluated and modeled using Chimera v1.11.2 software. Superposition and structural alignments were made using the Match Maker tool with Iteration no greater than 2 Å.
RESULTS
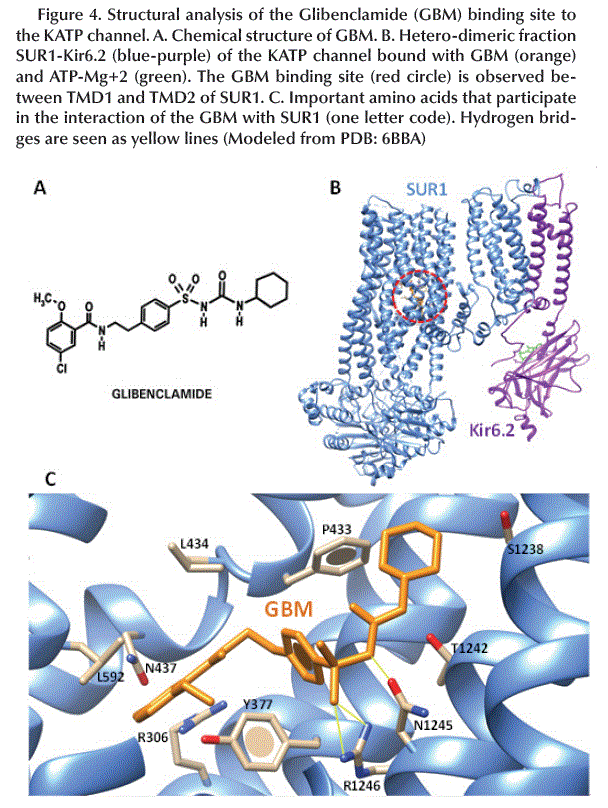
Binding site of GBM
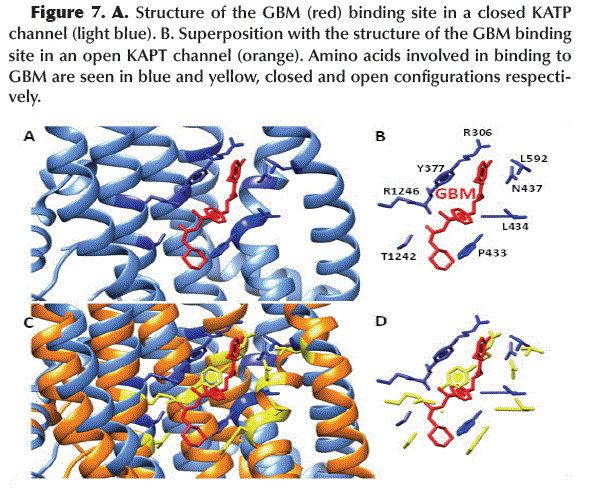
The chemical structure of GBM (Figure 4A) allows it to interact to the SUR1 subunit between the TMD1 and TMD2 domains in the KATP channel (Figure 4B). The amino acids involved in this interaction are mainly aromatic and hydrophobic such as Y377, P434, L434 and L592 and form hydrogen bonds between R1246/N1245 and -SO2/-NH- (Figure 4C).
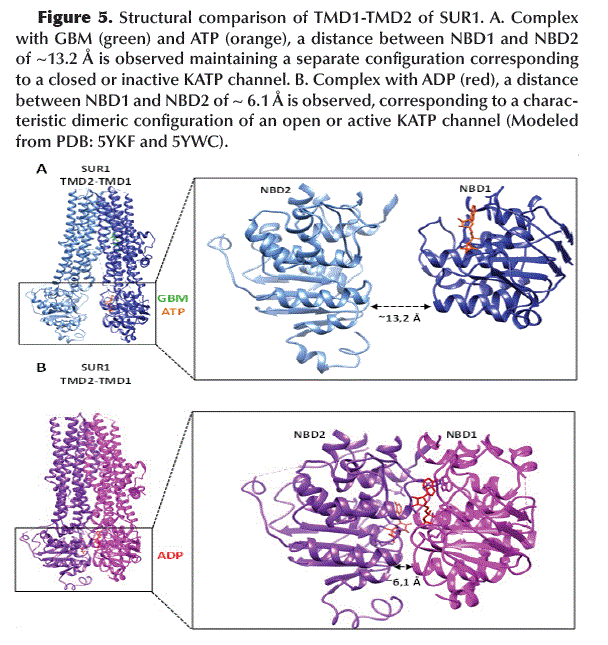
NBD1-NBD2 dimerization
Binding of GBM (with SUR1) and ATP (with NBD1) keeps the NBD1 and NBD2 domains (located at TMD1 and TMD2, respectively) in a separate configuration (~13.2 Å apart) corresponding to a closed or inactive KATP channel (Figure 5A). In contrast, binding of ADP to NBD1 and NBD2 promotes its dimerization, with both domains closest to each other (~6.1 Å apart) which is characteristic of an open or active KATP channel (Figure 5B).
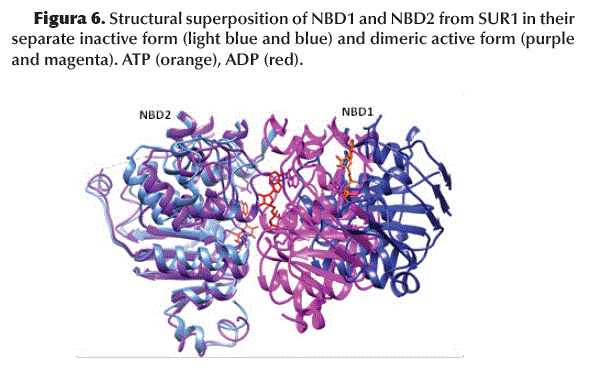
The superposition of the structures of NBD1 and NBD2 indicates an evident conformational change induced by the binding of GBM/ATP or ADP (Figure 6).
Occlusion of GBM binding site
After dimerization of NBD1 and NBD2 by the binding of ADP, a conformational change in SUR1 results in the narrowing of the GBM binding site, primarily by rotation of the Y377 and P433 residues (Figure 7), which maintains the KATP channel in its active or open configuration.
Binding site of PIP2 is blocked
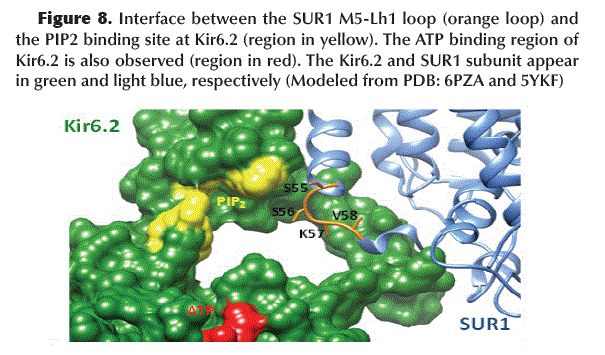
The binding of GBM to SUR1 induces a conformational change in the M5-Lh1 loop (S55, S56, K57 and V58) of TMD1-SUR1 acquiring a configuration that blocks the PIP2 binding site located in Kir6.2 (Figure 8).
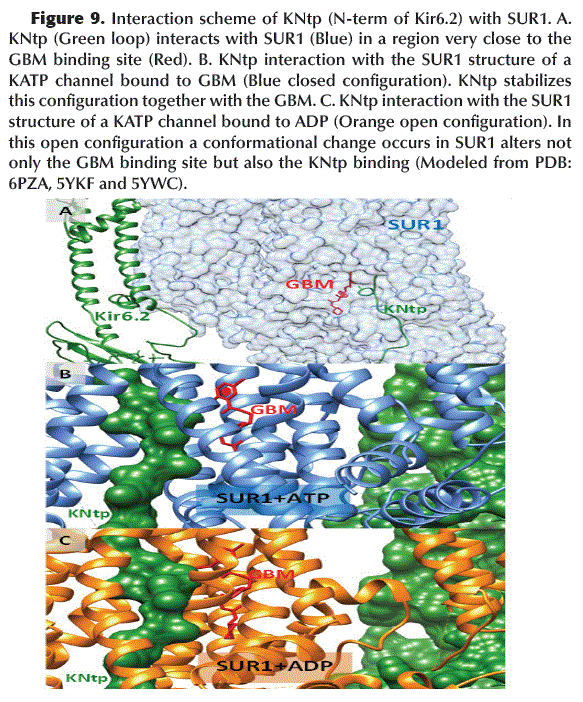
Role of KNtp in the activation of KATP channel
The N-terminal region of Kir6.2 (KNtp) interacts inside SUR1 very close to the GBM binding site (Figure 9A). Apparently, this interaction stabilizes the structure of the KATP channel in a closed (or inactive) configuration (Figure 9B), whereas, the dimerization of NBD1 and NBD2 (located in TMD1 and 2 of SUR1, respectively) after ADP binding, it causes a conformational change in SUR1 that is incompatible with the stabilizing interaction of KNtp and the binding of GBM in SUR1 (Figure 9C) causing the opening of the KATP channel.
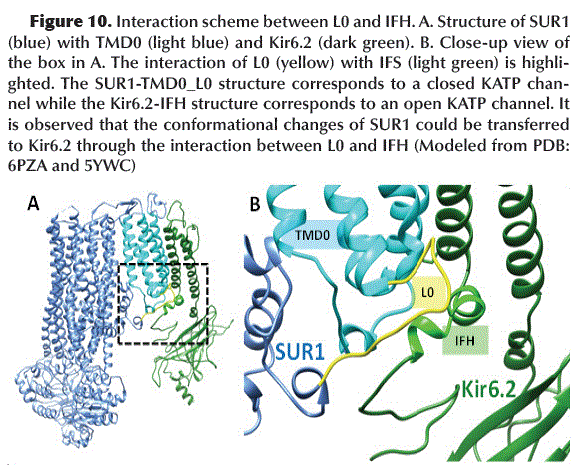
L0 and IFH communicate SUR1 with Kir6.2
The L0 loop present in the TMD0 of SUR1 in its inactive state (closed KATP channel) acquires a configuration that allows it to interact with the Kir6.2 IFH (Interface Loop) (Figure 10). This interaction probably promotes the Kir6.2 subunits (which shape the KATP channel pore) to acquire a closed configuration.
DISCUSSION
GBM is located in the interface between TMD0-L0 and TMD1-TMD2 acting as a wedge to stabilize the SUR1 structure in an inactive or closed conformation (with NBD1 and 2 separated) and also stabilizing the TMD0-L0 fragment in a conformation that could inhibit the opening of Kir6.2 (Li et al., 2017). A comparative study points out that in the presence of GBM and ATP-Mg2+ both the internal and external helices of the Kir6.2 TMD move inward giving rise to a channel with a narrow and closed helix input (channel inactive or closed).
All Kir6.2 residues that interact with SUR1 are located primarily before the helical structures that make up the pore of the KATP channel, the Kir6.2 fragments composed of the outer helices such as IFH and the N-terminal region (KNtp) they are sufficient to interact with SUR1. The Lasso Motif (present in the L0 interface) could act as a region that communicates the signal sent by the SUR1 regulatory subunits to the Kir6.2 subunits that are the ones that make up the channel per se, since in closed conformation, this motif interacts with IFH (Kir6.2 Interfacial Loop) possibly promoting a conformational change that results in the closure of the KATP channel. However, the Lasso Motif is only visible in the inactive (closed) but not in the active (open) configuration due to the low resolution and low electron density obtained in the crystallographic structures and the great mobility that this region has (Martin et al., 2019). Other studies indicate that when the KATP channel is in its active (open) form (called the "quatrefoil" state), the Lasso Motif acquires a disordered configuration representing a decoupled state between SUR1 and Kir6.2, in which the conformational change of SUR1 cannot be transferred to Kir6.2 or vice versa (Lee et al., 2017).
Other studies based on the microscopic technique of reconstruction of molecules on an atomic scale (Lee et al., 2017), allowed obtaining 3D reconstruction maps for the KATP channel of human pancreatic cells. The maps show that these channels have binding sites for ATP and ADP. The ADP binding site acts as a sensor that can detect small changes in cellular levels of ADP. In addition, a dynamic Loop-like structure was revealed that connects the ATP and ADP junction areas, this Motif plays an important role allowing the increase in ADP to cancel the action that ATP has on the KATP channel (since ADP and ATP have opposite effects). The presence of an ADP sensor and the Lasso Motif could explain how KATP channels monitor changes in the ATP/ADP ratio and can therefore control the release of Insulin based on blood Glucose levels. Genetic defects in the functioning of the KATP channels of the pancreas can cause many diseases (Nichols, 2006; Quan et al., 2011). Understanding the structure and function of these channels is essential for the discovery, analysis, or design of new drugs that serve to complement or improve the treatment of diseases such as T2DM.
CONCLUSION
The results obtained from the comparison of different crystallographic structures of the KATP channel from human pancreatic cells, support the activation model proposed by Wu et al., 2018, in which both GMB and KNtp (N-terminal Kir6.2) bind cooperatively within the SUR1 subunit and inhibit activation (opening) of the KATP channel, while ATP may be linked to NBD1 (degenerate site) but with separate NBD1/2 domains. In turn, the M5-Lh1 loop of SUR1 blocks the PIP2 binding site (which is a KATP channel activator) located at Kir6.2. When the ADP concentration increases, it first binds to NBD2 (consensus site) inducing a conformational change and increasing the affinity of NBD1 for ADP or ATP, promoting the closure and dimerization of both NBDs. Finally, a global conformational change of SUR1 occurs (due to NBD1/2 dimerization), which activates the tetrameric channel made up of Kir6.2 subunits (opening of the channel), blocking the GBM binding site and rearranging the conformation of KNtp. In the absence of GBM, KNtp can still remain bound to SUR1 but with less affinity.
REFERENCES
Aittoniemi, J., Fotinou, C., Craig, T.J., de Wet, H., Proks, P., and Ashcroft, F.M. (2009). Review. SUR1: a unique ATP-binding cassette protein that functions as an ion channel regulator. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364: 257–267. [ Links ]
Aguilar-Bryan, L., Bryan, J., Nakazaki, M. (2001). Of mice and men: k (ATP) channels and insulin secretion. Recent Progress in Hormone Research 56: 47–68. [ Links ]
Ashcroft, F. M. (2005). ATP-sensitive potassium channelopathies: focus on insulin secretion. Journal of Clinical Investigation 115: 2047–2058. [ Links ]
Hansen, S.B., Tao, X., and MacKinnon, R. (2011). Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477: 495–498. [ Links ]
Lee, K., Chen, J. and MacKinnon, R. (2017). Molecular structure of human KATP in complex with ATP and ADP. Biophysics and Structural Biology Neuroscience eLife 6:e32481. [ Links ]
Li, N., Wu, J. X., Ding, D., Cheng, J., Gao, N. and Chen, L. (2017). Structure of a Pancreatic ATP-Sensitive Potassium Channel. Cell 168: 101–110. [ Links ]
Martin, G. M., Yoshioka, C., Rex, E. A., Fay, J. F., Xie, Q., Whorton, M. R., Chen, J. Z. and Shyng, S. L. (2017). Cryo-EM structure of the ATP-sensitive potassium channel illuminates mechanisms of assembly and gating. Biophysics and Structural Biology Neuroscience eLife 2017;6:e24149. [ Links ]
Martin, G. M., Sung, M. W., Yang, Z., Innes, L. M., Kandasamy, B., David, L. L., Yoshioka, C. and Shyng, S. L. (2019). Mechanism of pharmacochaperoning in a mammalian KATP channel revealed by cryo-EM. Biophysics and Structural Biology Neuroscience eLife 8:e46417. [ Links ]
Nichols, C. G. (2006). KATP channels as molecular sensors of cellular metabolism. Nature 440: 470–476. [ Links ]
Quan, Y., Barszczyk, A., Feng, Z. P., and Sun, H. S. (2011). Current understanding of KATP channels in neonatal diseases: focus on insulin secretion disorders. Acta Pharmacol. Sin. 32: 765–780. [ Links ]
Vedovato, N., Ashcroft, F.M., and Puljung, M.C. (2015). The nucleotide-binding sites of SUR1: a mechanistic model. Biophys. J. 109: 2452–2460. [ Links ]
Wu, J. X., Ding, D., Wang, M., Kang, Y., Zeng, X. and Chen, L. (2018). Ligand binding and conformational changes of SUR1 subunit in pancreatic ATP-sensitive potassium channels. Protein Cell 9(6): 553–567. [ Links ]
Zhang, Z., and Chen, J. (2016). Atomic structure of the cystic fibrosis transmembrane conductance regulator. Cell 167: 1586–1597. [ Links ]