Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Journal of the Selva Andina Research Society
Print version ISSN 2072-9294On-line version ISSN 2072-9308
J. Selva Andina Res. Soc. vol.13 no.1 La Paz 2022
https://doi.org/10.36610/j.jsars.2022.130100035
Research Note
Comparison of in vitro anti-Staphylococcus aureus activity of eight antibiotics and four dilutions of propolis
1Development of the Ministry of Agriculture of Cuba. Reference Laboratory for Apicultural Research and Health (LARISA). Address: Esquina Carretera Circunvalante S/N, Sancti Spirítus. Cuba. Tel: 41375652-52992653. desarrollo@larisaulcsa.minag.gob.cu diagnostico@larisaulcsa.minag.gob.cu director@larisaulcsa.minag.gob.cu alayn.rivea@gmail.com
2Center for Molecular Immunology. Calle 216 esq. A 15, Atabey, Playa. Habana. Cuba. Tel: 76384460-53189615. lisania@cim.sld.cu
3Fructuoso Rodriguez Orthopedic Teaching Hospital. Address: F Street corner 29. Vedado. Revolution Square. Habana. Cuba. Tel: 53249870-78836655. alduque@infomed.sld.cu
4University of Sancti Spíritus "José Martí Pérez". Faculty of Agricultural Sciences. Cuba. Carretera Central, Esquina Paseo S/N juanemilio@uniss.edu.cu
El objetivo de esta investigación se basó en la comparación de halos de inhibición de una muestra de Staphylococcus aureus, enfrentada in vitro a ocho antibióticos de uso en medicina, así como, al enfrentamiento a cuatro concentraciones alcohólicas de propóleos. Los resultados señalan que la acción antimicrobiana de propóleos, depende de los compuestos bioactivos como: flavonoides, polifenoles, ácidos aromáticos, etc., contenidos en este producto. La actividad antibacteriana de las cuatro diluciones de propóleos, produjeron halos de inhibición entre 10 y 20 mm. De los ocho antibióticos con los que se realizó la comparación, solo la eritromicina resultó ser resistente a S. aureus. La penicilina produjo los halos con menores dimensiones. Aunque los halos derivados por el resto de los antimicrobianos fueron mayores a los del propóleos, muchos de ellos se encontraron dentro del rango originado por dicho producto de la colmena, no se ha demostrado resistencia antibacteriana comparada, con la que a lo largo de los años los antibióticos presentan, al ser usados indiscriminadamente para eliminar o controlar agentes antimicrobianos.
Palabras clave: Propóleos; extracto etanólico; antimicrobiano; halos; inhibición
The objective of this research was based on the comparison of inhibition halos of a sample of Staphylococcus aureus, faced in vitro with eight antibiotics used in medicine, as well as four alcoholic concentrations of propolis. The results indicate that the antimicrobial action of propolis, depending on the bioactive compounds such as: flavonoids, polyphenols, aromatic acids, etc., contained in this product. The antibacterial activity of the four propolis dilutions produced inhibition halos between 10 and 20 mm. Of the eight antibiotics with which the comparison was made, only erythromycin was found to be resistant to S. aureus. Penicillin produced halos with smaller dimensions. Although the halos derived from the rest of the antimicrobials were greater than those from propolis, many of them were found within the range originated by said product from the hive. Compared antibacterial resistance has not been demonstrated, with which over the year’s antibiotics present, when used indiscriminately to eliminate or control antimicrobial agents
Keywords: Propolis; ethanolic extract; antimicrobial; halos; inhibition
Introduction
Antibiotics constitute the treatment of infectious diseases. The development of evolutionary mechanisms, by microorganisms to evade antibiotic action, generates risks in therapy and health, both animal and human. Despite advances in research, search and development of new antimicrobials, difficult situations are frequently reported when treating bacterial infections that, due to their indiscriminate use, present resistance to them1.
In recent years, various microorganisms have developed resistance to different drugs used for their control. Among these, the high resistance of Escherichia coli and Staphylococcus aureus to antibiotics stands out, constituting the main causes of hospital-acquired illnesses2. S. aureus presents resistance to methicillin between 50 to 85 % of isolates worldwide3.
Staphylococci are Gram-positive aerobic microorganisms. The most pathogenic of them, S. aureus, typically causes skin infections, sometimes pneumonia, endocarditis, and osteomyelitis, and is generally associated with abscess formation. Some strains produce toxins that cause gastroenteritis, scalded skin syndrome, and toxic shock syndrome. Diagnosis is made with Gram stain and culture, treatment usually includes penicillinase resistant betalactams, but as resistance to antibiotics is common, the use of vancomycin or other more modern drugs may be necessary4.
Due to the high resistance of pathogens in Mexico against various treatments with synthetic antibiotics such as: ampicillin, ciprofloxacin, ceftriaxone (those that have shown susceptibility below 60 %), treatments of natural origin are sought. and friendly to the environment5.
Propolis is a natural and innocuous product used in traditional medicine since ancient times, due to its biological properties, a substance with a complex composition and resinous appearance, elaborated by Apis mellifera L. bees from the vegetation adjacent to the hive, these insects collect plant exudates, damaged tissues as a protection measure, resins, lacquers, gums and oils6.
The biological activity of propolis is due to its chemical composition based on: 55 % resins and balsams, 30-40 % beeswax, 5-10 % essential or volatile oils, 5 % pollen and other organic and mineral materials7. The resinous fraction of propolis is made up of phenolic and flavonoid compounds that are very important therapeutically8, they have an essential synergistic effect for beneficial biological activities in humans and animals: antibacterial, antifungal, antiviral, antioxidant, immunomodulatory, among others9.
Due to the antimicrobial capacity provided by polyphenols, mostly flavonoids, esters and phenolic acids, different beneficial effects for health are attributed to propolis10.
Other propolis compounds apart from polyphenols include: caffeic acid, ferulic acid, isoferulic acid, 3,4-dimethoxycinnamic acid, pinobanksin, caffeic acid benzyl ester, caffeic acid phenethyl ester, apigenin, pinocembrin, chrysin and galangin, etc11. Flavonoids, aromatic acids, diterpenoids and phenolic compounds are considered to be the main chemical constituents responsible for the biological properties of propolis12.
The antimicrobial activity, a fundamental property of propolis, its bacteriostatic, bactericidal action is based on the inhibition of nucleic acids and degradation of the cytoplasmic membrane, mainly attributed to flavonoids such as pinocembrin, quercetin, naringenin, acacetin, apigenin, chrysin, galangin, kaempferol and pinobanskina13, as well as alteration in ion channels as a result of phosphorylation and dephosphorylation reactions, decreasing the inhibition of bacterial motility.
Akca et al.14 state that the antimicrobial mechanisms shown by flavonoids are: inhibition of motility, inhibition of nucleic acid synthesis, inhibition of cytoplasmic membrane functions, inhibition of metabolism energy, inhibition of binding and biofilm formation, inhibition of porins, and attenuation of pathogenicity.
Flavonoids are compounds made up of two phenyl rings, A and B, linked through a pyran ring C with additional functional groups, which generates different types of flavonoids, such as chalcones, flavanones, flavones, flavonols, proanthocyanidins, anthocyanidins, flavandiols, isoflavones and aurones, compounds that give it antioxidant, antimicrobial, healing and anti-inflammatory characteristics15.
Apart from the active role that flavonoids play in the destruction of organisms, they strongly affect connective tissues by inhibiting some of the enzymes that can hydrolyze their network of proteoglycans and proteins. This mesh sterically hinders the diffusion of infectious organisms through the tissue16.
Given its antimicrobial properties, propolis is the subject of countless studies where the inhibitory effect on bacteria such as: E. coli, Lactobacillus plantarum, S. aureus, S. epidermidis, Pseudomonas aeruginosa, P. fluorescens, Listeria monocytogenes, L. innocua was evaluated, Klebsiella pneumoniae, Salmonella typhimurium, S. enteritidis, Streptococcus agalactiae, S. mutans, Bacillus cereus, B. subtilis, Citrobacter freundii, Enterobacter aerogenes, Shigella dysenteriae, Yersinia enterocolitica, Pantoea agglomerans, Vibrio cholerae, among others, the results are variable even when dealing with the same microorganism12,17.
In vitro tests have shown that propolis extracts are more effective against Gram-positive (S. aureus, Streptococcus β-haemolyticus) and that they only act against some Gram-negative bacteria such as E. coli or P. aeruginosa18.
Authors such as Gil et al.19 and Agurto Mendizábal & Cuya López20 obtained antimicrobial activity with a partial bacteriostatic and bactericidal effect by applying ethanolic extracts of Venezuelan, European and Mexican propolis20, against S. aureus, by using minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of 1 % and 2 %, and MIC of 62.5 μg/mL and 125 μg/mL.
Considering that the excessive use of antibiotics leads to antimicrobial resistance in bacteria, and it is necessary to study treatments of natural origin against them, the objective of this work was based on making a comparison of the inhibition halos of a sample of S. aureus after applying in vitro antibiogram discs of eight antibiotics used in health and discs of four ethanolic concentrations of propolis, in order to obtain satisfactory results with propolis solutions and thus have a natural and safe product to confront the microorganism analyzed.
Materials and methods
The S. aureus strain used in this research came from the strain bank belonging to the Center for Hygiene and Epidemiology of the province of Sancti Spíritus, Cuba, and was isolated from a patient with a skin lesion. In the Reference Laboratory for Research and Beekeeping Health (LARISA), the microorganism was planted in nutrient agar medium (AN), after incubation and growth at 37 ºC/24 h, it was kept refrigerated, until the moment of storage its use for research work, this bacterium was confronted with eight antibiotics, as well as four alcoholic dilutions of propolis. The propolis was purchased from the API-CUBA Company, in charge of marketing this product from the hive.
The dilutions of ethanolic extracts of propolis (EEP) were prepared with distilled water, alcohol and crude propolis according to the volume to be prepared and the amount in grams of said product that was needed for each dilution. To carry out the dilutions, the method described by Sforcin21 was taken into account, who used 30 g of propolis to elaborate a dilution in 100 mL of 70 % alcohol, although under laboratory conditions a sample of propolis (30 g), to which 100 mL of 96 % alcohol of this pure formulation was added, the different calculations of the dilutions with lower concentration of propolis and alcohol were made. After preparing the four solutions, they were kept in amber containers protected from light for 14 days. Several times a day all the dilutions were homogenized to help the propolis to contribute its bioactive properties in the ethanolic preparation. At the end of the period, the dilutions were purified on sterile filter paper, keeping the extracts in amber bottles at room temperature.
Table 1 Preparation of ethanolic extracts of propolis (EEP) or propolis tinctures
| EEP | 20% | 15% | 10% | 5% |
|---|---|---|---|---|
| Propolis (g) | 20 g/100 mL | 15 g/100 mL | 10 g/100 mL | 5 g/100 mL |
| Alcohol | 64º | 48º | 32º | 16º |
| Distilled water (mL) | 34 | 51 | 67 | 84 |
In the investigation, antibiogram disks of eight antibiotics used in the control of the bacteria named above were also used, they were provided by the Ministry of Public Health of Cuba, corresponding to the Brand Liofilchem, Italy with Lot number: 093019116.
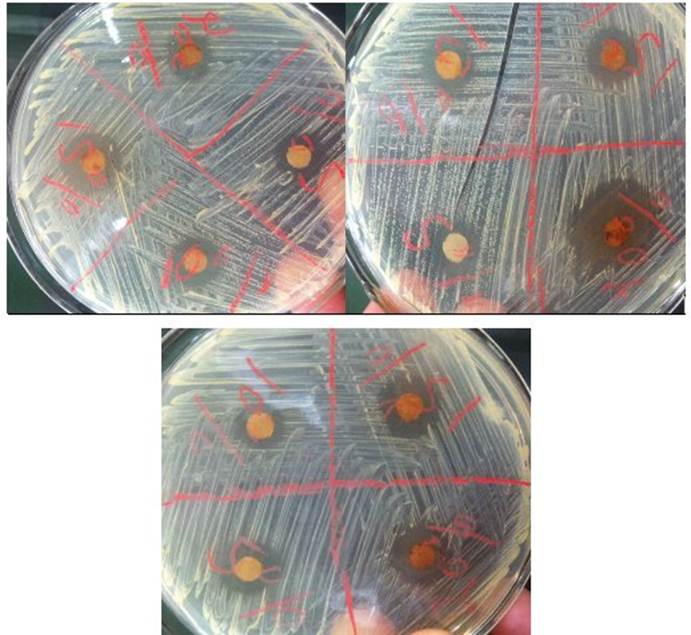
In the laboratory work, under sterile conditions inside a Biological Safety Cabinet, eighteen sterile Petri dishes were used with the AN culture medium, in each of the plates the sowing of S. aureus was carried out, then of taking a well loaded hoe from a previous crop. Bacterial colonies were streaked in the medium, performing this process across the width of the plates. With the help of a crystallographic pencil, each plate was divided into four. In each framed section, the antibiotic that would be used to verify the MIC of S. aureus, therefore, six plates contained the antibiogram discs for: amikacin, chloramphenicol, tetracycline, and penicillin, and another six with the discs for: doxycycline, ciprofloxacin, cotrimoxazole, and erythromycin. Filter paper discs previously sterilized and soaked for 24 h with propolis dilutions of 20, 15, 10 and 5 % were added to the remaining six plates. Then, all the plates were incubated at 37 ºC/24 h, after which the inhibition halos produced by the antibiotics were measured as the ethanolic dilutions of propolis.
Since Mueller Hilton agar culture medium (MHA) was not available in the laboratory, an ideal medium for reading the antibiogram, AN was used, where the growth and inhibition of S. aureus was also verified.
To carry out the statistical analysis of the results, Statgraphics Version 5.1 in Spanish for Windows22 was used, the Contrast Tests t of comparison of means (t-test) were carried out to know if there were significant differences in the results obtained for the different percentages at which propolis tinctures were applied.
Results
Table 2 Inhibition halos and means obtained by the antibiotics, after the reading made after 24 h of incubation
| Antibiotic | amikacin | chlorampfenicol | tetracycline | penicillin | doxycycline | ciprofloxacin | cotrimoxazole | erytromycin |
|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 16 | 18 | 10 | 19 | 23 | 20 | 0 |
| 2 | 20 | 20 | 17 | 7 | 20 | 21 | 23 | 0 |
| 3 | 16 | 22 | 17 | 12 | 21 | 21 | 20 | 0 |
| 4 | 20 | 24 | 19 | 12 | 24 | 23 | 23 | 0 |
| 5 | 17 | 27 | 19 | 9 | 20 | 23 | 25 | 0 |
| 6 | 16 | 24 | 19 | 3 | 21 | 20 | 11 | 0 |
| Promedio | 18.1666667 | 22.16666667 | 18.16666667 | 10.5 | 20.833333 | 21.83333333 | 20.16666667 |
Table 3 Halos of inhibition and means obtained by the ethanolic extracts of propolis after the reading made
| EEP (%) | 20 | 15 | 10 | 5 |
|---|---|---|---|---|
| 1 | 15 | 15 | 14 | 11 |
| 2 | 15 | 13 | 12 | 10 |
| 3 | 16 | 16 | 13 | 14 |
| 4 | 15 | 17 | 13 | 14 |
| 5 | 20 | 13 | 12 | 10 |
| 6 | 20 | 15 | 12 | 12 |
| Average | 16.8333333 | 14.83333333 | 12.6666667 | 11.8333333 |
Of the eight antibiotics used, antimicrobial activity against S. aureus was obtained with seven of them, erythromycin did not show inhibition (Table 2).
The four ethanolic dilutions of propolis gave halos of inhibition against the microorganism to a greater or lesser extent. Although with most antibiotics, halos higher than those obtained with propolis solutions were reached, their halos presented similarities to those produced by antibiotics, in addition, when comparing propolis dilutions at 10 and 5 %, we obtained inhibition halos greater than those reported by penicillin (Table 3). Statistical analysis between antibiotics revealed differences that mainly benefited chloramphenicol, doxycycline, ciprofloxacin, and cotrimoxazole. Amikacin and tetracycline showed highly significant differences when compared to penicillin.
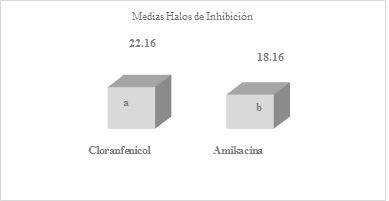
Figure 1 Means obtained by averaging the inhibition halos of the six replicates of amikacin and chloramphenicol antibiotics, after the readingsDifferent letter s express significant difference, benefiting chloramphenicol with a P-Value = 0.0470679
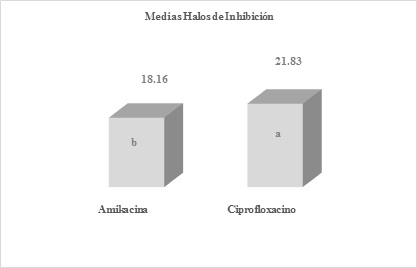
Figure 2 Means obtained by averaging the inhibition halos of the six replicates of amikacin and ciprofloxacin antibiotics, after the readingsDifferent letters express significant difference, benefiting ciprofloxacin with a P-Value = 0.00419613
The means obtained by averaging the inhibition halos of the six replicates of antibiotics amikacin and chloramphenicol (Figure 1), amikacin and ciprofloxacin (Figure 2), cotrimoxazole and penicillin (Figure 3), after readings after 24 hours of incubation. As mentioned before, when comparing the halos produced by amikacin and tetracycline, with those of penicillin, highly significant differences were found with values of p=0.000105644 and 0.0000178965, against S. aureus.
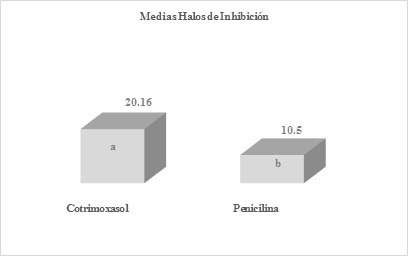
Figure 3 Means obtained by averaging the inhibition halos of the six replicates of cotrimoxazole and penicillin antibiotics, after the readingsDifferent letters express significant difference, benefiting cotrimoxazole with a P-Value= 0.00132035
In Figure 4, the inhibition halos against S. aureus are due to the antimicrobial action of the antibiotics, as well as the absence of halos by erythromycin.
During the statistical analysis of the four ethanolic concentrations of propolis, differences were found between the 20-10, 20-5, 15-10 and 15-5 % dilutions, with the 20 and 15 % solutions benefiting. Comparing propolis tinctures with antibiotics, differences favoring most antibiotics were obtained. Between the 20 % dilution and amikacin, tetracycline, cotrimoxazole, no statistical differences were found as they had similar results in terms of the inhibition halos of the bacteria studied. Were only reached significant differences when confronting the propolis concentrations of 20 and 15 %, and comparing them with the results obtained by penicillin (P= 0.000947989 and 0.00329998). Although the dilutions 10 and 5 % showed inhibition halos greater than those produced by this last antibiotic, they did not show statistical differences.
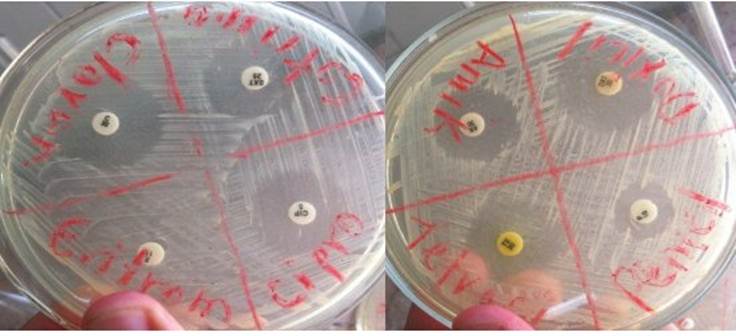
Figure 4 Halos of inhibition of Staphylococcus aureus produced by seven of the antibiotics used in the researchAs can be seen, the bacteria is also inhibited in nutrient agar medium, in the absence of Mueller Hilton agar medium.
In Figures 5 and 6, the results of the means obtained by averaging the inhibition halos of the six replicates of propolis at 20 and 10 % (Figure 5), propolis at 15 and 5 %, (Figure 6) after the readings after 24 h of incubation.
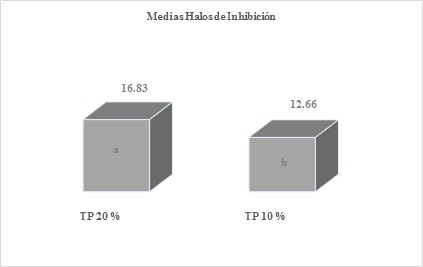
Figure 5 Means obtained by averaging the inhibition halos of the six replicates of propolis at 20 and 10 %, after the readingsDifferent letters express significant statistical difference, benefiting the 20 % solution with a P-Value= 0.00294017
In addition to these analyzes between the ethanolic solutions of propolis, significant statistical differences were also obtained when comparing the 20-5 and 15-10 % tinctures, with the 20 and 15 % dilutions being favored at all times.
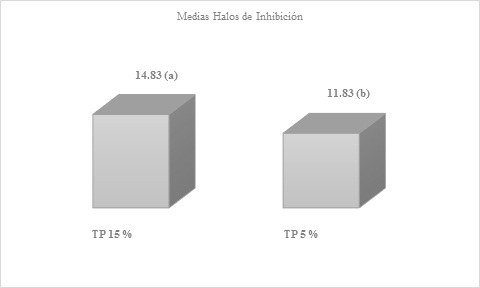
Figure 6 Means obtained by averaging the inhibition halos of the six replicates of propolis at 15 and 5 %, after readingsDifferent letters express significant statistical difference, benefiting the 15 % solution with a P-Value= 0.012966
The inhibition produced by the four ethanolic solutions of propolis can be clearly seen, which could have been greater, if kept in incubation for 24 more hours, but it was decided.
Discussion
García Rodríguez et al.23 expose in a previous work, the standard patterns of the inhibition halo for S. aureus against a large number of antibiotics, some of these were used in this research: penicillin (resistant to halos ≤ 28 mm, there is no range for intermediate, while the bacterium would be sensitive to halos ≥ 29 mm) , erythromycin (resistant halos ≤ 13 mm, intermediate 14-22 and sensitive ≥ 23 mm), ciprofloxacin (resistant halos ≤ 15 mm, intermediate 16-20 and sensitive ≥ 21 mm), chloramphenicol (resistant halos ≤ 12 mm, intermediate 13-17 and sensitive ≥ 18 mm), tetracycline (resistant halos ≤ 14 mm, intermediate 15-18 and sensitive ≥ 19 mm). When analyzing the averages reached by some of the antibiotics used in this study, after comparing them with the standard patterns of the inhibition halo proposed by García Rodríguez et al.23, Staphylococcus spp. shows sensitivity to ciprofloxacin and chloramphenicol, while against tetracycline its inhibition falls to the intermediate range.
According to Mensa et al.24, more than 90 % of S. aureus isolates produce betalactamases that inactivate penicillin, one of the reasons being that in said investigation penicillin caused less inhibition of S. aureus. Koneman et al.25 suggest that S. aureus develops resistance to antibiotics such as penicillin and erythromycin, our results coinciding with those stated by said authors.
The antimicrobial activity of propolis is attributed to its compounds: phenolic acids, coumarins, alkaloids, flavonoids, esters, phenolic aldehydes and ketones, a high antimicrobial potential, antibiotic, fungicidal, antiviral and antitumor action, among others26-28. The presence of pentacyclic triterpenes constitutes an important characteristic of the chemical composition of propolis, linked to its anti-inflammatory, anticancer and antiviral activity29. Boisard et al.30 in in vitro antimicrobial studies with propolis revealed that the inhibitory activity against Gram-positive bacteria belonging to the Staphylococcus and Streptococcus genera is given by pinocembrin, a flavonoid present in said hive product. According to Popova et al.31, A. honey bees produce propolis rich in totarol and totarolone diterpenes, which have shown antimicrobial activity against S. aureus. Trusheva et al.32 state that propoline C, D, F and G inhibit the growth of Gram-positive bacterial strains (S. aureus, B. subtilis, L. monocytogenes and Paenibacillus larvae), showing propoline C, the greater antibacterial activity against the bacteria studied, which was resistant to methicillin, showing that Gram-positives have high susceptibility to the action of the active principles of propolis, since the cell wall of these bacteria It is made up of only one type of molecule compared to Gram-negative bacteria, which have a multilayered wall, and by therefore, they are more resistant to the antimicrobial activity of said hive product33.
The results achieved with the EEP solutions show greater inhibition halos and also on a smaller scale than those obtained by Ferreira Bastos et al.34, in their research they applied sixteen propolis samples against S. aureus, reaching inhibition halos between 8 and 23 mm. Data similar to those of this research were reported by Kujumgiev et al.35, in a study of the antimicrobial activity of propolis from different countries, they obtained inhibition halos between 11 and 29 mm for S. aureus. Tovalino & Contreras36, in vitro investigated the antibacterial effect of two propolis solutions from Oxapampa-Peru (10 and 30 %) against S. aureus (ATCC 25923), at 30 % it showed greater inhibition with a mean of 11.77 mm. In this investigation, superior results were obtained with the four dilutions used. Gómez et al.37, used EEP concentrations of 20, 40, 60, 80 and 100 % (96 % alcohol dilutions), against S. aureus in which they reached halos between 13 and 17 mm, determining the 20 % dilution as the one with the highest MIC. Cabrera-Rojas38 used propolis ethanolic solutions between 90, 80, 70 and 60 % against the bacteria studied, and obtained inhibition halos of 28.5 mm, 27.5 mm, 17.1 mm and 8.5 mm. The 90 and 80 % dilutions caused the largest halos, and were higher than those obtained in our work, although, with the 70 and 60 % concentrations used by the author, they obtained halos that coincide with those obtained in this research, and also lower, being demonstrated that, with lower solutions, the inhibition of said microorganism is achieved. Bucio-Villalobos & Martínez-Jaime39, obtained inhibition halos of 1.6 cm with EEP. Gil et al.19 in their research using the macro dilution technique in a tube showed that S. aureus can be inhibited with EEP at much higher concentrations drops of 2 and 1 %. Agurto Mendizábal & Cuya López20 reached inhibition halos of 12 mm when using EEP from Ayacucho and Huaral (Mexico), against S. aureus. The same results were obtained in this work with dilutions of 10 and 5 %, in which halos of 13 and 14 mm were also observed.
Díaz-Mena et al.40, when evaluating the antimicrobial activity of Cuban EEP by the agar dilution method, showed that the EEP had a markedly greater effect against Gram-positive bacteria, with MIC values between 0.025 and 10 %, coinciding their results with those of our research, inhibition of the growth of S. aureus, Gram-positive, was obtained with a concentration of 10 %. Other researchers such as Mercan et al.41 and Gonsales et al.42 found antibacterial activity in propolis samples from Turkey, Panama and Sao Paulo, against Gram-positive bacteria, such as S. aureus.
According to Moreno et al.43, during their research with propolis dilutions, when S. aureus is incubated for 48 h, its bactericidal effect is increased. In their study, they concluded that 70 % of the propolis samples increased their antimicrobial activity after an incubation time of 48 h when compared to the effect detected at 24 h. As in this investigation the readings for both the antibiotics and the propolis dilutions were made at 24 h of incubation, this time could have influenced that greater inhibition halos were not obtained for the propolis concentrations, if they had been maintained at incubation for two days.
Taking into consideration the research of Bucio-Villalobos & Martínez-Jaime39 who verified that when using ethanol as a control treatment against S. aureus, said reagent did not cause any inhibitory effect, compared to the inhibition caused by the EEP, it was decided not to apply it as another control treatment, in this work.
EEP can be an alternative in antibacterial treatments, since it is a natural product to which resistance has not been demonstrated and whose only contraindication until now is that the patient is allergic to bee products44.
Of the eight antibiotics used in the investigation, only erythromycin did not produce inhibition of S. aureus in comparison with the four ethanolic dilutions of propolis, inhibition of the bacteria was obtained with all solutions. Compared to the patterns with which the inhibition halos produced by the antibiotics were compared, the microorganism was sensitive to ciprofloxacin and chloramphenicol, while its inhibition fell to the intermediate range when compared to tetracycline. The antimicrobial action of penicillin was inactivated by the betalactamases produced by this microorganism when it came into contact with this antibiotic. The concentrations of propolis 20 and 15 % caused the greatest halos of inhibition of the microorganism by this product of the hive. During the statistical analysis, both concentrations showed significant differences when comparing the halos produced by them, with those obtained with the antibiotic penicillin. Although the halos derived from the EEP of 10 and 5 % were greater than those of penicillin, no statistical difference was obtained when comparing them with said antibiotic. All propolis solutions showed inhibition of the analyzed microorganism and, although in this work the presence of its bioactive compounds was not verified as cited by some authors, it can be estimated from the results achieved that the Cuban propolis has these biological properties.
REFERENCES
1. Resistencia a los antimicrobianos [Internet]. Organización Mundial de la Salud. 2020 [citado 5 de marzo de 2021]. Recuperado a partir de: http://www.who.int/mediacentre/factsheets/fs194/es/ [ Links ]
2. Cabrera CE, Gómez RF, Zúñiga AE. La resistencia de bacterias a antibióticos, antisépticos y desinfectantes una manifestación de los mecanismos de supervivencia y adaptación. Colomb Méd 2007;38(2):149-58. [ Links ]
3. Gil de MM. Staphylococcus aureus: Microbiología y aspectos moleculares de la resistencia a meticilina. Rev Chil Infectol 2000;17(2):145-52. DOI: https://doi.org/10.4067/S0716-10182000000200010 [ Links ]
4. Portillo ME, del Pozo JL. Infecciones por estafilococo. Medicine 2018;12(49):2890-4. DOI: https://doi.org/10.1016/j.med.2018.02.002 [ Links ]
5. Reyes-Acevedo M, Toro V, Chávez G, Lagos-Hernández R, Godoy-Cumillaf A, Caniuqueo-Vargas A. Vigilancia de la resistencia bacteriana en instituciones de salud de la ciudad de Hermosillo, Sonora, México. Salud Pública Méx 2018;60 (2):117-8. DOI: https://doi.org/10.21149/8446 [ Links ]
6. Palomino García LR, Martínez Galán JP, García Pajón CM, Gil González JH, Durango Restrepo DL. Caracterización fisicoquímica y actividad antimicrobiana del propóleos en el municipio de la unión (Antioquia, Colombia). Rev Fac Nac Agron Medellín 2010;63(1):5373-83. [ Links ]
7. Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol 2011;133(2):253-60. DOI: https://doi.org/10.1016/j.jep.2010.10.032 [ Links ]
8. Rodríguez Pérez B, Canales Martínez MM, PenieresCarrillo JG, Cruz Sánchez TA. Composición química, propiedades antioxidantes y actividad antimicrobiana de propóleos mexicanos. Acta Univ 2020;30:e2435. DOI: https://doi.org/10.15174.au.2020.2435 [ Links ]
9. Soto-Vásquez MR. Metabolitos secundarios, cuantificación de fenoles y flavonoides totales de extractos etanólicos de propóleos de tres localidades del Perú. Crescendo 2015;6(2):22-32. [ Links ]
10. Da Cunha MG, Franchin M, de Carvalho Galvão LC, de Ruiz AL, de Carvalho JE, Ikegaki M, et al. Antimicrobial and antiproliferative activities of stingless bee Melipona scutellaris geopropolis. BMC Complement Altern Med 2013;13:23. DOI: https://doi.org/10.1186/1472-6882-13-23 [ Links ]
11. Li A, Xuan H, Sun A, Liu R, Cui J. Preparative separation of polyphenols from water-soluble fraction of Chinese propolis using macroporous absorptive resin coupled with preparative high performance liquid chromatography. J Chromatogr B Biomed Appl 2016;1012-1013:42-9. DOI: https://doi.org/10.1016/j.jchrom.2015.12.038 [ Links ]
12. Siripatrawan U, Vitchayakitti W, Sanguandeekul R. Antioxidant and antimicrobial properties of thai propolis extracted using ethanol aqueous solution. Int J Food Sci Technol 2013;48(1):22-7. DOI: https://doi.org/10.1111/j.1365-2621.2012.03152.x [ Links ]
13. Vargas-Sánchez R, Torrescano-Urrutia GR, Mendoza-Wilson AM, Vallejo-Galland B, Acedo-Félix E, Sánchez-Escalante JJ, et al. Mecanismos involucrados en la actividad antioxidante y antibacteriana del propóleos. Biotecnia 2014;16(1):32-7. DOI: https://doi.org/10.18633/bt.v16i1.31 [ Links ]
14. Akca AE, Akca G, Topçu FT, Macit E, Pikdöken L, Özgen IS. The comparative evaluation of the antimicrobial effect of propolis with chlorhexidine against oral pathogens: An in vitro study. Biomed Res Int 2016;2016:3627463. DOI: https://doi.org/10.1155/2016/3627463 [ Links ]
15. Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci 2016;5:e47. DOI: https://doi.org/10.1017/jns.2016.41 [ Links ]
16. Farnesi AP, Aquino-Ferreira R, De Jong D, Bastos JK, Soares AE. Effects of stingless bee and honey bee propolis on four species of bacteria. Genet Mol Res 2009;8(2):635-40. DOI: https://doi.org/10.4238/vol8-2kerr023 [ Links ]
17. Osés SM, Pascual-Maté A, Fernández-Muiño MA, López-Díaz TM, Sancho MT. Bioactive properties of honey with propolis. Food chem 2016;196:1215-23. DOI: https://doi.org/10.1016/j.foodchem.2015.10.050 [ Links ]
18. Mayta-Tovalino F, Sacsaquispe Contreras S, Ceccarelli Calle J, Alania Mallqui J. Propóleo peruano: Una nueva alternativa terapéutica antimicrobiana en Estomatología. Rev Estomatol Hered 2012;22(1):50-8. DOI: https://doi.org/10.20453/reh.v22i1.159 [ Links ]
19. Gil M, Gonzalez M, Orlandi O, Ugas K, Nicita G, Perozo E. Actividad bacteriostática y bactericida de extractos etanólicos de propóleos venezolanos y europeos sobre Escherichia coli y Staphylococcus aureus. MedULA 2018;25(1):6-12. [ Links ]
20. AgurtoMendizábal JC, Cuya López E. Actividad antimicrobiana del extracto etanólico liofilizado de propóleo procedente de Ayacucho y Huaral frente a Staphylococcus aureus y Pseudomonas aeruginosa [tesis licenciatura]. [Lima]: Universidad Nacional Mayor de San Marcos; 2021 [citado 26 de octubre de 2021]. Recuperado a partir de: https://cybertesis.unmsm.edu.pe/handle/20.500.12672/16527 [ Links ]
21. Sforcin J. Efeito da sazonalidade sobre as propriedades imunomoduladora e antibacteriana da própolis e perfil bioquímico de ratos [tesis doctoral]. Botucatu: Universidade Estadual Paulista Botucatu; 1996. [ Links ]
22. Statgraphics 19 centurion [Internet]. Statgraphics 19 centurion. 2000 [citado 5 de marzo de 2021]. Recuperado a partir de: https://www.statgraphics.com/ [ Links ]
23. García Rodríguez JA, Cantón R, García Sánchez JE, Gómez-Lus MªL, Martínez Martínez L, Rodríguez-Avial C, et al. Métodos básicos para el estudio de la sensibilidad a los antimicrobianos. En: Picazo JJ, editor. Procedimientos en Microbiología Clínica [Internet]. Granada: Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica; 2000. p. 1-54. Recuperado a partir de: https://www.seimc.org/contenidos/documentoscientificos/procedimientosmicrobiologia/seimc-procedimientomicrobiologia11.pdf [ Links ]
24. Mensa J, Soriano A, Llinares P, Barberán J, Montejo M, Salavert M, et al. Guía de tratamiento antimicrobiano de la infección por Staphylococcus aureus. Rev Esp Quimioter 2013;26(Suppl 1): S1-S84. [ Links ]
25. Koneman E, Allen S, Janda W, Schreckenberger P, Winn Jr W. Diagnostico Microbiologico: Ed. Médica Panamericana. 2008:71. [ Links ]
26. Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem Toxicol 1998;36(4):347-63. DOI: https://doi.org/10.1016/s0278-6915(97)00145-2 [ Links ]
27. Duke CC, Tran VH, Duke RK, Abu-Mellal A, Plunkett GT, King DI, et al. A sedge plant as the source of Kangaroo Island propolis rich in prenylated p-coumarate ester and stilbenes. Phytochemistry 2017;134:87-97. DOI: https://doi.org/10.1016/j.phytochem.2016.11.005 [ Links ]
28. Delgado Aceves MdL, Andrade Ortega JÁ, Ramírez Barragán CA. Caracterización fisicoquímica de propóleos colectados en el Bosque La Primavera Zapopan, Jalisco. Rev Mex Cienc Forestales 2015;6(28):74-87. [ Links ]
29. Ito J, Chang FR, Wang HK, Park YK, Ikegaki M, Kilgore N, et al. Anti-AIDS agents. 48.(1) Anti-HIV activity of moronic acid derivatives and the new melliferone-related triterpenoid isolated from Brazilian propolis. J Nat Prod 2001;64(10):1278-81. DOI: https://doi.org/10.1021/np010211x [ Links ]
30. Boisard S, LeRay A-M, Landreau A, Kempf M, Cassisa V, Flurin C, et al. Antifungal and antibacterial metabolites from a French poplar type propolis. Evid Based Complement Alternat Med 2015;2015:319240. DOI: https://doi.org/10.1155/2015/319240 [ Links ]
31. Popova MP, Chinou IB, Marekov IN, Bankova VS. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry 2009;70(10): 1262-71. DOI: https://doi.org/10.1016/j.phytochem.2009.07.025 [ Links ]
32. Trusheva B, Popova M, Koendhori EB, Tsvetkova I, Naydenski C, Bankova V. Indonesian propolis: chemical composition, biological activity and botanical origin. Nat Prod Res 2011;25(6):606-613. DOI: https://doi.org/10.1080/14786419.2010.488235 [ Links ]
33. Akca AE, Akca G, Topçu FT, Macit E, Pikdöken L, Özgen IS. The Comparative evaluation of the antimicrobial effect of propolis with Chlorhexidine against oral pathogens: An in vitro study. Biomed Res Int 2016;2016:3627463. DOI: https://doi.org/10.1155/2016/3627463 [ Links ]
34. Ferreira Bastos EM, Guzmán D, Figueroa J, Tello J, Scoaris DdO. Caracterización antimicrobiana y fisicoquímica de muestras de propóleos proveniente de Apis mellifera L. (Hymenoptera: Apidae) la Región Andina Colombiana. Acta Biol Colomb 2011;16(1):175-84. [ Links ]
35. Kujumgiev A, Tsvetkova I, Serkedjieva Y, Bankova V, Christov R, Popov S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol 1999;64(3):235-40. DOI: https://doi.org/10.1016/s0378-8741(98)00131-7 [ Links ]
36. Tovalino FRM, Contreras SS. Evaluación in vitro del efecto antibacteriano del extracto etanólico de propóleo de Oxapampa-Perú sobre cultivos de Streptococcus mutans (ATCC 25175) y Staphylococcus aureus (ATCC 25923). Rev Estomatol Hered 2010;20(1):19-24. DOI: https://doi.org/10.20453/reh.v20i1.1777 [ Links ]
37. Gómez J, Peña N, Pérez C, Gutiérrez-Cortez C, Suarez Mahecha H. Evaluación por dos métodos in vitro de actividad antimicrobiana de propóleos frente a algunos microorganismos de interés alimentario. Rev Fac Nal Agr Medellín 2014;67 (Suppl 2):131-4. DOI: https://doi.org/10.13140/2.1.4919.3920 [ Links ]
38. Cabrera-Rojas LAJ. Eficacia antimicrobiana in vitro del extracto etanólico de propóleo sobre cepas de Staphylococcus aureus [tesis licenciatura]. [Trujillo]: Universidad César Vallejos; 2016 [citado 26 de octubre de 2020]. Recuperado a partir de: https://repositorio.ucv.edu.pe/handle/20.500.12692/550 [ Links ]
39. Bucio-Villalobos CM, Martínez-Jaime OA. Actividad antibacteriana de un extracto acuoso de propóleo del municipio de Irapuato, Guanajuato, México. Agron Mesoam 2017;28(1);223-7. DOI: https://doi.org/10.15517/am.v28i1.24253 [ Links ]
40. Díaz-Mena D, Gil Rodríguez JD, Valdés González G. Determinación de la CMI de propóleos cubanos a partir de dos técnicas diferentes. Apiciencia 2000;2(2):1-12. [ Links ]
41. Mercan N, Kivrak I, Duru ME, Katircioglu H, Gulcan S, Malci S, et al. Chemical composition effects onto antimicrobial and antioxidant activities of propolis collected from different regions of Turkey. Ann Microbiol 2006;56(4):373-8. DOI: https://doi.org/10.1007/BF03175035 [ Links ]
42. Gonsales GZ, Orsi RO, FernandesJúnior A, Rodrigues P, Funari SRC. Antibacterial activity of propolis collected in different regions of Brazil. J Venom Anim Toxins Incl Trop Dis 2006;12(2): 276-84. DOI: https://doi.org/10.1590/S1678-91992006000200009 [ Links ]
43. Moreno Z, Martínez P, Figueroa J. Efecto antimicrobiano in vitro de propóleos argentinos, colombianos y cubano sobre Streptococcus mutans ATCC 25175. Nova 2007;5(7):70-5. DOI: https://doi.org/10.22490/24629448.376 [ Links ]
44. Gil M, Perelli A, Alvarado R, Arias Y, Blumenthal E. Actividad bacteriostática y bactericida de la tintura de propóleos sobre bacterias enteropatógenas. Salus 2012;16(3):21-5. [ Links ]
Funding source For the work, no source of financing was used, since the materials, reagents, media, products (propolis) were found in advance in the laboratory and it was not necessary to use funds for its acquisition.
Acknowledgments We appreciate the technical help of colleagues María de los Ángeles Leal del Ojo Blanco and Adnelis Delgado Montiel, who also participated in the clinical trial, and without their support it would have been difficult to carry it out. The authors thank all those people interested in research that contributes to a common good, such as improving human health. We also thank the institutions and publishers that allow these works to reach the hands of all, and that are also useful for practical use in pursuit of health.
Ethical considerations This is a job that was carried out with the greatest respect and professionalism, in order to find solutions to the indiscriminate use of antibiotics with which the resistance of bacteria towards them has been seen, and in this way apply natural products such as propolis to which the microorganisms have not developed resistance and which, moreover, would help control germs such as S. aureus.
Authors' contribution to the articleKen Jact Fernández León, José Antonio Rodríguez Díaz, worked on the search for bibliographies and during the laboratory investigation, sowing S. aureus in the culture medium, as well as adding the different antibiogram disks and those embedded in the solutions ethanolic propolis, also in the reading and measurement of the inhibition halos after the incubation period and the execution of the statistical analyses. Lisandra Reyes Espinosa and Amílcar Duquesne Alderete, provided the antibiogram discs, and participated in the preparation and revision of the work together with Ken Jact Fernández León and José Antonio Rodríguez Díaz, Yovanni Solenzal Valdivia, Alayn Rives Quintero and Juan Emilio Hernández García, participated in the bibliography search and in the creation of the graphs.
Received: April 01, 2021; Revised: July 01, 2021; Accepted: December 01, 2021











 text in
text in