Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Científica Ciencia Médica
versión impresa ISSN 2077-3323
Rev Cient Cienc Méd vol.21 no.1 Cochabamba 2018
Original article
GENERIC DRUGS, PERCEPTION OF THE DOCTORS. CALI, COLOMBIA
Camilo Torres Serna1, Juan Camilo Ángel Medina2, Helen Viviana Klinger Torresb, Vanessa Márquez Flórezb, Jean Marcos Micolta Bejaranob, Jhon Jairo Sánchez Suescúnb.
Programa de Medicina, Universidad Libre, Cali-Colombia
1 Médico-Farmacólogo, PhD en Ciencias de la Educación. Investigador del Instituto de Investigaciones Biomédicas de la Universidad Libre y Profesor Titular de Farmacología, Universidad Santiago de Cali. Colombia.
2 Estudiante, Programa de Medicina, Universidad Libre, Seccional de Cali, Colombia.
E-mail: catorse@yahoo.com
Accepted for publication: May, 20 2018
Cite as: Rev Cient Cienc Med 2018;21(1):40-49
ABSTRACT
It is common to hear medical mistrust of the effectiveness of generic drugs. The objective of this study is to quantify the acceptance of physicians linked to the General System of Social Security in Health of Colombia to the prescription of generic drugs.
Was performed a cross-sectional, descriptive study. Eighty doctors in clinical practice were interviewed, all doctors who made clinical consultation in the practice sites of a Medical School of Cali-Colombia. The results show that the physicians consulted are clear about the concept of generic medicine and 89.6% of them consider the generic product essentially similar to the original reference medicine, but at the same time, they tend to think that generic products do not comply with the desired therapeutic effect. It is also stand out that 74,4% of doctors consider that patients value the use of generic products little.
Keywords: prescription of drugs, generic drugs, innovative medicines, fraudulent drugs.
INTRODUCTION
The management and administration of medication use is a determining factor of financial equilibrium of health organization. They have constantly stablish and view bid processes, purchasing, distribution and adequate supplies of medication to users and this area represents a great potential administrative inquiry. Around the world exist a great controversy about innovative drugs and their analogues also known as generic drugs1,2. Public health entities generally prefer to buy generic products, for the most part unexpensive, but doctors and patients think they are ineffective products3,4. A generic drug is potentially similar to the first investigational medicine, with a chemical composition called innovative drug5,6. A research laboratory develops an innovative molecule with therapeutic purposes and then, it starts the marketing of brand-name drugs, this molecule has been registered by an intellectual property patent for twenty years, afterwards other laboratories start producing and marketing the same molecule as International Nonproprietary Name (INN) or other brand-names. These products, with the same composition and misnamed copy drugs, while generic drugs is the right name. In some countries, they are denominated as interchangeable medicines (Mexico). World Health Organization (WHO) recommend using the term Multisource drugs7.
The objective of the study was to identify the concept and opinion about generic drugs of doctors of Sistema General de Seguridad Social en Salud (SGSSS) of Colombia, which gives healthcare to 90% of Colombian people8. We expect to obtain information in order to target educational campaigns and provide sustained information with figures on bioequivalence for the medicinal products and the importance of reaching a rationalization in drug use from medical staff, pharmacists and patients; moreover, to contribute to expensive pharmacologic substances control and avoid the unnecessary use.
MATERIALS AND METHODS
It was performed a cross-sectional, descriptive study. To pick up information, it was used a mixed questionnaire known as PAMPEFG-01 (Percepción y Actuación de Médicos en la Prescripción de Especialidades Farmacéuticas Genéricas), a valid instrument used by García AJ et al , and adapted for this study.
The universe involves all general practitioners and specialists that accomplished inclusion criteria: working in direct healthcare services (emergency services and outpatient basis) at clinical practice areas of Faculty of Medicine of Cali-Colombia University, people who didn't work in medical area and develop only administrative work were excluded from the study. This found that seven clinical practice areas of level 1, 2 and 3 with medical complexity. Only 245 general practitioners and specialists from different shifts, via different forms of contract (full-time, half-time or eventual service delivery) accomplished the inclusion criteria. It was applied a sample size calculator software (Rotator Survey®), and obtained a minimum sample of 70 practitioners, which were selected through a probability sampling method the appointed day. We interview each doctor, allocating the time needed and confidentiality to answer the questionnaire.
This research takes part from a large project about social drug use performed by Health Management Group of Health Management Program and the Biomedical Research Institute of Universidad Libre of Cali-Colombia. The participants of the study got information about research purposes and an informed consent that specified voluntary participation of people.
According to the Standards of Health Ministry of Colombia, it was deemed a risk-free research because any biological, physiological, psychological or social intervention of the participants in this study was performed. A survey was carried out, in which the identification of participants weren't revealed due to no personal information or behavior sensitive aspects were requested. The Ethical and Scientific Committee for research involving humans of Universidad Libre de Cali approved, the initiation of this study by means of minutes signed 05,2016.
RESULTS
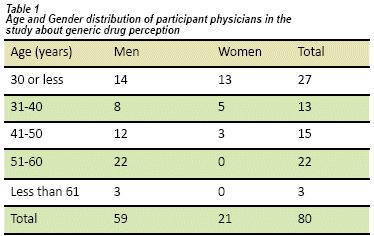
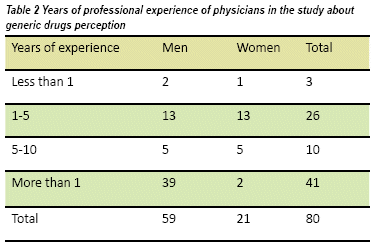
We interviewed 80 clinically active faculty physicians, of which 59 were men and 21 women, from emergency services and outpatient basis. Fifty-three were >31 years old (see table 1), 41 were general practitioners and 39 had a specialty. Half of the physicians had 10 years of work experience and only three had less than one year (see Table 2).
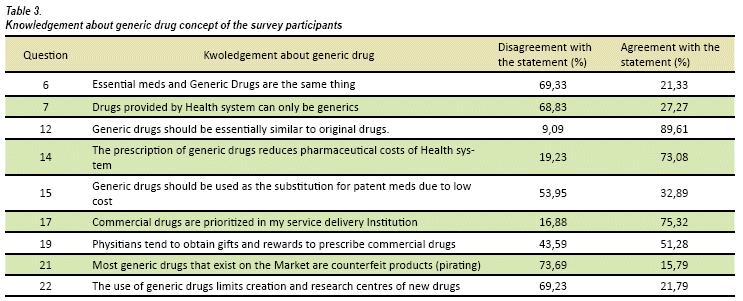
All the questions on the form were analyzed by knowledge of physicians in relation to generic drug concept (see Table 3) and physician confidence in generic drugs (see Table 4). The answers show good knowledge of generic drug concept, 69% recognize the difference between an essential drug (WHO defined, as those that satisfy the priority health care needs of the population) and a generic drug; 73% considers not to confuse a generic drug with a counterfeit drug. The 89,6% accounts generic are similar to original drugs and 69% doesn't believe the generic drug use can limit pharmacological research.
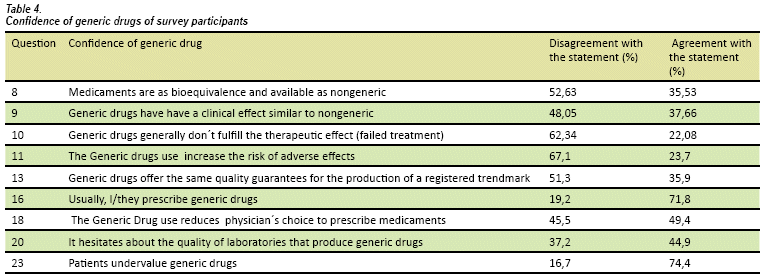
In respect of questions regarding confidence physician of generic drugs (see Table 4), 71,8% use generic drugs, 62,3% didn't find treatment failure and 67,1% doesn't consider an increase of adverse effects. A crucial result is interviewed physicians perceive patients undervalued generic drugs use.
The following Table 4 summarizes the knowledge of generic drug concept of interviewed physicians, it compares agreement and disagreement answers, and uncertain responses were ruled out.
It's important for health system actors to have clear concepts about myths and facts of generic drugs11. The term generic, doesn't mean a pharmaceutic product put on the market under the name of the active molecule, as many people believe, this term refers to a drug, which isn't originally develop by a pharmaceutical company, but made by others12, there is overwhelming evidence of chemical, pharmaceutic and clinical bioequivalence between original and generic products13,14.
The results of this study present interviewees have a clear concept of generic drug (see table 3), 89,6% consider a generic product must be essentially similar to original drug, at the same time, 62,3% think generic products comply with the desired, therapeutic effect. We emphasized 74,4% consider patients underestimate generic drugs use.
Despite generic drugs are as effective as brand-name drugs and cheaper, the study's results and others show a significant proportion of doctors (around 40% in this study), that prefer original products and think patients do, too. Dr. Óscar Andia, Director of Monitoring Drug of Colombian Medical Federation (Observamed), present Colombia
has a fake and deep-rooted thought about generic drugs are worst, low quality cited by Silva S15. Including doctors prefer to recommend this option.
According to Instituto Colombiano de Vigilancia de Medicamentos y Alimentos (Invima), generic drugs are produced with high standards of quality and cost less because they aren't included patent, development and research costs, moreover, manufacturers spend less in advertising stuff16.
A meta-synthesis study compared the use of generic medicine and non-generic (innovative or original products) in key medical situations, they are mentioned below.
In Universidad Del Valle, Cali, Colombia, compared the bioavailability of two high engagement products, original cyclosporine immunosuppressant with a generic drug17 and between low molecular weight heparin (original enoxaparin) and a generic drug. Both of either them, didn't present significant differences in measured pharmacokinetic parameters, or area under the curve (AUC), or maximum concentration (Cmax), or time required to achieve maximum concentration (Tmax)18.
Kesselheim, Misono et al, performed a systematic search of publications in MEDLINE, EMBASE and International Pharmaceutical Abstracts, there were 47 scientific papers about detailed knowledge that covered nine different subclasses of cardiovascular drugs, 81% represented randomized clinical trials.
The clinic equivalent was tested in seven encountered beta-blockers trials, in 10 of the 11 reviewed about diuretics trials, in five of seven calcium antagonists, in three about antiplatelet agents, in two reviews of statins, in one of ACE inhibitors and other about alpha-blockers. This meta-analysis also analyzed 53 publishers and opinions, in which expressed a negative point of view about generic drugs compared with 28% that encourage innovative to generic drugs replacement.
The substitution of generic drugs of antihypertensive medication doesn't lead to less compliance instead of, increases cardiovascular hospital admissions compared with innovative drugs therapy. When a generic antihypertensive is available, it must be considered the original drug replacement in order to achieve economic benefits and ensure chronic treatment21.
In other medical specialties, there are studies as Vlahiotis y Devine22 with similar results, it describes major depressive episode patients, in which didn't observed high treatment abandon rate between the ones that start with generic therapy and the ones with original meds.
One of the biggest controversies reported in literature, is the substitution of brand name for generic drugs, in people with epilepsy. Polard et al, studied the association between generic therapy replacement and the loss of crisis control, a total of 8 379 patients were analyzed along with sensitivity analysis yielded similar results. Authors concluded that the substitution of anticonvulsants for generic drugs are not associated with high risk of seizures.
Pereira et al, made the comparison of the blood-thinning effect of oral warfarin based on International Normalized Ratio effect, there weren't differences between original and generic meds that forced to a dose setting. Neither they also found difference biological variation of INR based on warfarin formulation (p> 0,69), nor interaction between warfarin and patient (p> 0,81). The conclusion of this studies is that patients can change from original warfarin for generic with security and effectiveness.
As Duerden and Hughes raised, despite the substitution of innovative products for their generic can affect some individual patients, specially the reduction of adherence and high potential medication errors, it might be a price worth paying due to a huge opportunity for money savings, associated with drugs use clinically no better compared with the cheap alternatives.
The insurance companies and pharmaceutical policy makers promote generic drugs use in order to reduce costs, but they continue currently underused. Physicians and patients agree generic drugs are as safe and cheaper.
The 74,4% of physicians consider patients rarely value generic drugs, which has been particularly significant. This finding tally with other studies27,28, as Shrank found that 56% of Americans think, they should use more generic drugs, only 37,6% prefer to take them29. There are actions of health officials, in attempting to change people thought, around the world. According to WHO, it's described that pharmaceutical industry has made great efforts in order to discredit generic drugs. "The usual is to stigmatize generic drugs showing them as an alternative for 'poor people' and low quality"30, States must do the same with this practices.
In conclusion, many studies31,32 consider generic drugs are as effectiveness and safe as original meds, and their introduction and marketing assume pharmaceutical money savings, which make them a necessary step to Health System sustainability33,34.
It's important to take into account counterfeit products, which aren't subject of study in this research. Under no circumstances, these products comply with conditions to be a generic drug, therefore it has to identify the origin of generic drug for ensuring its bioequivalence35,36.
In order to reduce patients and physicians susceptibility about generic drug use, it should spend time by giving information of medicament rational use to patients and prescribers. It's essential that physicians and pharmacists understand correctly the substitution of original meds for generic drugs approved by local authorities, and inform accurately to patients37.
Results from this and other researches appear to show, more efforts are necessary in order to Colombians trust in generic drugs and save a huge amount of resources to Health System.
Acknowledgements
The authors thanks to the Biomedical Research Institution, the Research group of Universidad Libre de Cali (Category A of Colciencias) for the logistics support received to complete this scientific paper.
Conflict of interests
All authors are involved actively in the elaboration of this manuscript, data collecting, analysis and drafting, and certify that they have NO affiliations with or involvement in any pharmaceutical industry, organization or entity with any financial interest.
REFERENCES
1. McLachlan AJ. Frequently asked questions about generic medicines. Aust Prescr [Internet].2007 (citado 5 de octubre de 2017); 41-3. Disponible en: https://cdn0.scrvt.com/08ab3606b0b7a8ea53fd0b40b1c44f86/fc20fd821a02cf80/8d2289edb5e3/ef-59843b3f060818930abb15d5ff4853aaeed461243034ea30c5d77ac475.pdf
2. Velásquez G. Pascale P; Organización Mundial de la Salud. Globalización y acceso a los medicamentos: Perspectivas sobre el acuerdo Adpic/Omc. 2 ed.; 2000. Disponible en: http://apps.who.int/medicinedocs/pdf/whozip47s/whozip47s.pdf
3. Consumers’ Association. Generic medicines: can quality be assured?. Drug Ther Bull. 1997; 35(2): 9–11.
4. Gaither C, Kirking D, Ascione F, Welage L. Consumers’ views on generic medications. J Am Pharm Assoc[Internet].2001(citado 22 de marzo de 2017); 41: 729–36. Disponible en: https://www.sciencedirect.com/science/article/pii/S1086580216313080?via%3Dihub
5. World Health Organization, Action Programme on Essential Drugs. Global comparative pharmaceutical expenditures. Geneva: WHO; 1997.
6. Al-Jazairi AS, Bhareth S, Eqtefan IS, Al-Suwayeh SA. Brand and generic medications: are they interchangeable?. Ann Saudi Med.2008; 28(1):33–41
7. OMS. Medicamentos Esenciales. Organización Mundial de la Salud, Ginebra, 2007
8. Espinosa O, Pinzón D. Análisis cuantitativo de la estacionalidad del gasto en salud en Colombia. Rev Monitor Estratégico [Internet].2018 (citado 10 de febrero de 2018); 13: 20. Disponible en: https://revistas.udem.edu.co/index.php/economico/article/view-File/2365/2003
9. García AJ, Martosa F, Leivab F, Sánchez de la Cuesta F. Genéricos: ¿buenos o malos? Conocimientos y actitudes de los médicos ante los medicamentos genéricos. Gac Sanit [Interenet]. 2003;17(2):144-9. Disponible en: http://www.scielo.br/pdf/gs/v17n2/breve.pdf
10. Isaza S. Siete verdades sobre medicamentos genéricos en Colombia. 1er. Foro nacional “La verdad sobre los medicamentos genéricos”. Bogotá, 2009 [Citado 15 de noviembre 2017]. Disponible en: http://colegiomedico.cundibogota.googlepages.com/FMC_PonenciaSenado21abr09FINAL.doc
11. Bolaños VH, D’Alessio R, Fefer E, de Joncheere K, Holzhauser de Uzcátegui E. Armonización de la reglamentación farmacéutica en América Latina. Washington, DC: Organización Panamericana de la Salud; 1996.
12. Davit BM, Nwakama PE, Buehler GJ, Conner DP, Haidar SH, Patel DT. et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43(10):1583-97.
13. Meredith P. Bioequivalence and other unresolved issues in generic drug substitution. Clin Ther.2003;25(11):2875–90.
14. Silva S. Los colombianos no saben comprar acetaminofén. [Citado 12 de mayo 2018]. Disponible en: https://www.elespectador.com/noticias/salud/colombianos-no-saben-comprar-acetaminofen-articulo-754430
15. Grupo de comunicaciones del Invima. Los medicamentos genéricos y los originales de marca tienen los mismos estándares de calidad. Instituto Nacional de Vigilancia de Medicamentos y Alimentos de Colombia. noviembre 2016. Disponible en: https://www.invima.gov.co/los-medicamentos-gen%C3%A9ricos-y-los-originales-de-marca-tienen-los-mismos-est%C3%A1ndares-de-calidad.html
16. Torres C, Rubiano J, Caicedo LA, Carvajal R. Bioequivalencia de dos presentaciones comerciales de ciclosporina oral. Anuario de Investigaciones 2000, Escuela de Medicina, U. del Valle. Cali, 2000.
17. Torres C, Vargas B, Noguera A, Rodríguez M, Vargas M, García O, et al. Estudio de biodisponibilidad comparativa y bioequivalencia de dos presentaciones de enoxaparina sódica. [Internet]. 2006 [Citado 15 de noviembre 2017]. Disponible en: http://www.susmedicos.com/0_Articulos_ General/Art_enoxaparina-sodica.html
18. Kesselheim AS, Misono AS, Lee JL, Stedman MR, Brookhart MA, Choudhry NK, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300:2514-26.
19. Van Wijk LG, Klungel OH, Heerdink ER, de Boer A. Generic substitution of antihypertensive drugs: does it affect adherence?. Ann Pharmacotherapy.2006;40:15-20.
20. Vlahiotis A, Devine S, Eichholz J, Kautzner A. Discontinuation Rates and Health Care Costs in Adult Patients Starting generic Versus Brand SSRI or SNRI Antidepressants in Commercial Helth Plans. J Managed Care Pharmacy. 2011;17:65-87
21. Polard E, Nowak A, Happe A, Biraben E. Brand-name to generic substitution of antiepileptic Drugs (AED) does not lead to seizure-related hospitalization: a Population-based case-crossover study. Clinical Therapeutics. 2015;37:e114.
22. Pereira JA, Holbrook AM, Dolovich L, Goldsmith C, Thabane L, Douketis JD, et al. Are brand-name and generic warfarin interchangeable?. Ann Pharmacother. 2005;39(7–8):1188–1193
23. Duerden MG, Hughes DA. Generic and therapeutic substitutions in the UK: are they a good thing?. British J Clin Pharm. 2010;70:335-41.
24. Meredith PA. Generic drugs: therapeutic equivalence. Drug Saf .1996;15(4):233–242.
25. Calvert RT. Bioequivalence and generic prescribing. J Pharm Pharmacol. 1996;48(1):9–10
26. Shrank WH, Cox ER, Fischer MA, Mehta J, Choudhry NK. Patients’ perceptions of generic medications. Health Aff (Millwood). 2009;28(2):546-56.. PubMed PMID: 19276015; PubMed Central PMCID: PMC2748784. Disponible en: doi: 10.1377/hlthaff.28.2.546
27. OMS. Comisión sobre determinantes sociales de la salud. Informe final. WHO/OMS [Internet]. 2009 [Citado 20 de enero 2018]. Disponible en: www.who.int/social_determinants/thecommission/finalreport/es
28. Verbeeck RK, Kanfer I, Walker RB. Generic substitution: the use of medicinal products containing different salts and implications for safety and efficacy. Eur J Pharm Sci. 2006; 28 (1–2): 1-3
29. Meyer MC. Generic drug product equivalence. Am J Manag Care. 1998;4(8):1183–92.
30. Bhosle, Deepak Sayyed, Asif; Bhagat, Abhijeet; Shaikh, Huzaif; Sheikh, Alimuddin; Bhopale, Vasundhara; Quazi, Zubair. Comparison of Generic and Branded Drugs on Cost Effective and Cost Benefit Analysis. Ann Int medical and Dental Research. 2016;3(1):35-7. Disponible en: doi: 10.21276/aimdr.2017.3.1.PC1
31. Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing. JAMA. 2007;298(1):61–9.
32. OMS. Medicamentos falsificados-Pautas para la formulación de medidas para combatir los medicamentos falsificados. Geneva, World Health Organization.1999. Disponible en: https://www.paho.org/hq/dmdocuments/2013/GCFM-Antecedentes-esp.pdf
33. Murphy JE. Generic substitution and optimal patient care. Arch Intern Med.1999;159(5):429–33
34. Kobayashi E, Karigome H, Sakurada T, Satoh N, Ueda S. Patients’ attitudes towards generic drug substitution in Japan. Health Policy. 2011;99:60-5.
5. World Health Organization, Action Programme on Essential Drugs. Global comparative pharmaceutical expenditures. Geneva: WHO; 1997. [ Links ]
6. Al-Jazairi AS, Bhareth S, Eqtefan IS, Al-Suwayeh SA. Brand and generic medications: are they interchangeable?. Ann Saudi Med.2008; 28(1):33–41
7. OMS. Medicamentos Esenciales. Organización Mundial de la Salud, Ginebra, 2007
8. Espinosa O, Pinzón D. Análisis cuantitativo de la estacionalidad del gasto en salud en Colombia. Rev Monitor Estratégico [Internet].2018 (citado 10 de febrero de 2018); 13: 20. Disponible en: https://revistas.udem.edu.co/index.php/economico/article/view-File/2365/2003
9. García AJ, Martosa F, Leivab F, Sánchez de la Cuesta F. Genéricos: ¿buenos o malos? Conocimientos y actitudes de los médicos ante los medicamentos genéricos. Gac Sanit [Interenet]. 2003;17(2):144-9. Disponible en: http://www.scielo.br/pdf/gs/v17n2/breve.pdf
10. Isaza S. Siete verdades sobre medicamentos genéricos en Colombia. 1er. Foro nacional “La verdad sobre los medicamentos genéricos”. Bogotá, 2009 [Citado 15 de noviembre 2017]. Disponible en: http://colegiomedico.cundibogota.googlepages.com/FMC_PonenciaSenado21abr09FINAL.doc
11. Bolaños VH, D’Alessio R, Fefer E, de Joncheere K, Holzhauser de Uzcátegui E. Armonización de la reglamentación farmacéutica en América Latina. Washington, DC: Organización Panamericana de la Salud; 1996.
12. Davit BM, Nwakama PE, Buehler GJ, Conner DP, Haidar SH, Patel DT. et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43(10):1583-97.
13. Meredith P. Bioequivalence and other unresolved issues in generic drug substitution. Clin Ther.2003;25(11):2875–90.
14. Silva S. Los colombianos no saben comprar acetaminofén. [Citado 12 de mayo 2018]. Disponible en: https://www.elespectador.com/noticias/salud/colombianos-no-saben-comprar-acetaminofen-articulo-754430
15. Grupo de comunicaciones del Invima. Los medicamentos genéricos y los originales de marca tienen los mismos estándares de calidad. Instituto Nacional de Vigilancia de Medicamentos y Alimentos de Colombia. noviembre 2016. Disponible en: https://www.invima.gov.co/los-medicamentos-gen%C3%A9ricos-y-los-originales-de-marca-tienen-los-mismos-est%C3%A1ndares-de-calidad.html [ Links ]
16. Torres C, Rubiano J, Caicedo LA, Carvajal R. Bioequivalencia de dos presentaciones comerciales de ciclosporina oral. Anuario de Investigaciones 2000, Escuela de Medicina, U. del Valle. Cali, 2000. [ Links ]
17. Torres C, Vargas B, Noguera A, Rodríguez M, Vargas M, García O, et al. Estudio de biodisponibilidad comparativa y bioequivalencia de dos presentaciones de enoxaparina sódica. [Internet]. 2006 [Citado 15 de noviembre 2017]. Disponible en: http://www.susmedicos.com/0_Articulos_ General/Art_enoxaparina-sodica.html
18. Kesselheim AS, Misono AS, Lee JL, Stedman MR, Brookhart MA, Choudhry NK, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300:2514-26. [ Links ]
19. Van Wijk LG, Klungel OH, Heerdink ER, de Boer A. Generic substitution of antihypertensive drugs: does it affect adherence?. Ann Pharmacotherapy.2006;40:15-20. [ Links ]
20. Vlahiotis A, Devine S, Eichholz J, Kautzner A. Discontinuation Rates and Health Care Costs in Adult Patients Starting generic Versus Brand SSRI or SNRI Antidepressants in Commercial Helth Plans. J Managed Care Pharmacy. 2011;17:65-87 [ Links ]
21. Polard E, Nowak A, Happe A, Biraben E. Brand-name to generic substitution of antiepileptic Drugs (AED) does not lead to seizure-related hospitalization: a Population-based case-crossover study. Clinical Therapeutics. 2015;37:e114. [ Links ]
22. Pereira JA, Holbrook AM, Dolovich L, Goldsmith C, Thabane L, Douketis JD, et al. Are brand-name and generic warfarin interchangeable?. Ann Pharmacother. 2005;39(7–8):1188–1193
23. Duerden MG, Hughes DA. Generic and therapeutic substitutions in the UK: are they a good thing?. British J Clin Pharm. 2010;70:335-41. [ Links ]
24. Meredith PA. Generic drugs: therapeutic equivalence. Drug Saf .1996;15(4):233–242.
25. Calvert RT. Bioequivalence and generic prescribing. J Pharm Pharmacol. 1996;48(1):9–10
26. Shrank WH, Cox ER, Fischer MA, Mehta J, Choudhry NK. Patients’ perceptions of generic medications. Health Aff (Millwood). 2009;28(2):546-56.. PubMed PMID: 19276015; PubMed Central PMCID: PMC2748784. Disponible en: doi: 10.1377/hlthaff.28.2.546
27. OMS. Comisión sobre determinantes sociales de la salud. Informe final. WHO/OMS [Internet]. 2009 [Citado 20 de enero 2018]. Disponible en: www.who.int/social_determinants/thecommission/finalreport/es
28. Verbeeck RK, Kanfer I, Walker RB. Generic substitution: the use of medicinal products containing different salts and implications for safety and efficacy. Eur J Pharm Sci. 2006; 28 (1–2): 1-3
29. Meyer MC. Generic drug product equivalence. Am J Manag Care. 1998;4(8):1183–92.
30. Bhosle, Deepak Sayyed, Asif; Bhagat, Abhijeet; Shaikh, Huzaif; Sheikh, Alimuddin; Bhopale, Vasundhara; Quazi, Zubair. Comparison of Generic and Branded Drugs on Cost Effective and Cost Benefit Analysis. Ann Int medical and Dental Research. 2016;3(1):35-7. Disponible en: doi: 10.21276/aimdr.2017.3.1.PC1 [ Links ]
31. Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing. JAMA. 2007;298(1):61–9.
32. OMS. Medicamentos falsificados-Pautas para la formulación de medidas para combatir los medicamentos falsificados. Geneva, World Health Organization.1999. Disponible en: https://www.paho.org/hq/dmdocuments/2013/GCFM-Antecedentes-esp.pdf [ Links ]










 texto en
texto en