Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta Nova
On-line version ISSN 1683-0789
RevActaNova. vol.7 no.2 Cochabamba Sept. 2015
ARTÍCULO CIENTÍFICO
On the synonymy of Nitzschia frustulum var. subsalina, N. inconspicua and N. boliviana sensu Trobajo et al. 2013
Sobre la sinonimia de Nitzschia frustulum var. subsalina, N. inconspicua and N. boliviana sensu Trobajo et al. 2013
Eduardo A. Morales
Herbario Criptogámico Universidad Católica Boliviana "San Pablo", Carrera de Ingeniería Ambiental. Casilla de Correos 5381, Cochabamba, Bolivia.
e-mail: edu_moral23@outlook.com
Recibido: 17 de noviembre 2015
Aceptado: 15 de diciembre 2015
In 2013, Trobajo and collaborators published an important contribution to the clarification of the taxonomy of some taxa of Nitzschia Hassall belonging to the Section Lanceolatae. This constitutes a group of relatively small species and infraspecific taxa that are common in freshwater samples and that are also commonly misidentified in routine ecological and floristic works. The importance of the Trobajo et al. paper is exacerbated by the inclusion of light (LM) and electron microscopy (SEM) data on type material of Nitzschia frustulum var. subsalina Hustedt, N. inconspicua Grunow and N. boliviana E.Morales & Vis (among others). Revision of type material is a necessary step in the clear definition of taxonomic and ecological boundaries, information that can be later used to define, for example, biogeographical distributions and usefulness of taxa in bioindication [11] [14] [15].
After comparison of LM and SEM images of N. frustulum var. subsalina, N. inconspicua and N. boliviana, Trobajo et al. [23] concluded that the three were conspecific and that they should be put in synonymy under N. inconpicua as the oldest validly published name. There are, however, some differences in the type population of N. boliviana, originally described from the Yungas of La Paz, Bolivia [13], which were not taken into account by Trobajo and collaborators. In the present manuscript, I discuss these differences in support of a separation of N. boliviana from other species in the Section Lanceolatae.
Table 1 shows a comparison among the taxa treated here. Data in this table were gathered from, and are circumscribed to, type material presented in Trobajo et al. [23] and Morales & Vis [13]. Before comparisons are made, it is convenient to first delimit well the range of variability of N. boliviana in type material. In plate 2 of the supplementary information, Trobajo et al. [23] present a size diminution series for N. boliviana from type material that is incorrect. The first and largest valve on the right of the upper row clearly does not belong to N. boliviana and does not appear to have been considered during calculations of size ranges (see their table 1). The more acutely-ended apices and the features of the raphe canal and fibulae in this specimen are different from the rest of the members in the mentioned series.
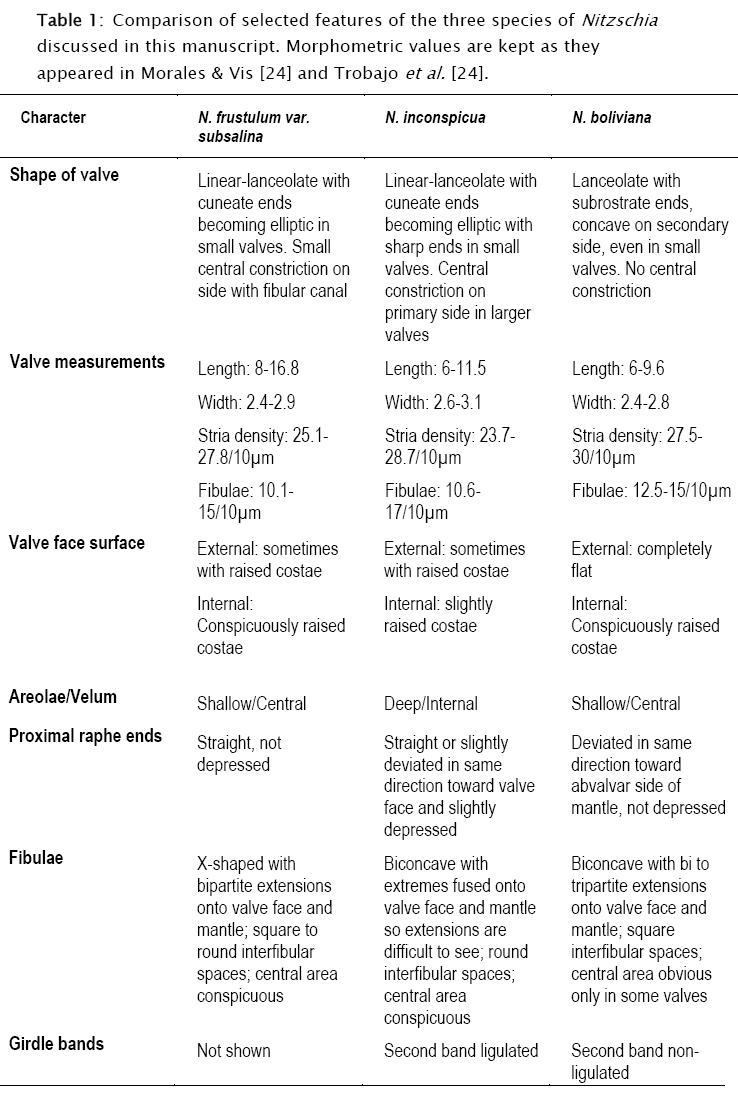
All characters considered in Table 1 are relevant in taxonomic treatments of Nitzschia spp. as shown by Mann [10]. The three taxa cannot be entirely separated based on valve measurements since there are overlaps in ranges for length, width, and density of stria and fibulae. Following Geissler [3] [4], Wendker & Geissler [25], Trobajo et al. [20] and Trobajo & Cox [21], these features are unreliable for defining affinities or taxonomy within Nitzschia since such features vary with environmental conditions such as salinity, turbulence and nutrient concentrations.
Looking at other features in Table 1, however, it is possible to find differences in N. boliviana when compared with the other two taxa. First, shape of valve is never linear and its apices are characteristically concave on the secondary side of the valve, a feature that is maintained even in small valves (figs 260-264 in Morales & Vis [13]; figs 15-17, and píate 2, figs 2-20 in Trobajo et al. [23]). At the central area of the valve there is no constriction on the primary side, the proximal terminations of the raphe are both deflected towards the abvalvar side of the mantle and there is no external depression (figs 271-276 in Morales & Vis [13]; figs 80-83 and plate 2, figs 21-22 in Trobajo et al. [23]). From the same figures it can be seen that the external surface of the valves is fíat, while in internal view the raised costae are clearly observable. Also, it is observed that the velum is placed in a very shallow position with respect to the valve external surface. The fibulae are biconcave with extensions onto valve face and mantle that can be bi or tripartite. The interfibular spaces are square and the central area is obvious only in some valves (figs 260-264 in Morales & Vis [13]; figs 15-17 and plate 2, figs 2-20 in Trobajo et al. [23]). Finally, the second girdle band does not possess a ligula (fig. 274 in Morales & Vis [13]; fig. 82 in Trobajo et al. [23]).
Some of the above features are different from those of N. inconspicua or N. frustulum var. subsalina, and some others are different from those in both taxa. From Table 1, it is also clear that N. frustulum var. subsalina and N. inconspicua have differences between them, raising questions about their conspecificity.
The features included in Table 1 and used to differentiate the three taxa included in this discussion are rather stable as shown by micrographs of the type populations. This stability allows the conclusion that the three populations can indeed be separated into three discriminate sets. In the absence of culturing and molecular data, something can still be said regarding the reliability of these distinguishing features. Some features of the raphe, central nodule, girdle bands, position of velum, shape of fibulae and distance between fibulae at the central area have been used to delimit species boundaries. Shape of valves and constrictions at the central area could be variable, but are often used as support characters to define species.
Though the distal ends of the raphe in nitzschioids has been shown to vary from valve to valve during formation of the frustule (e.g., Pickett-Heaps [16]), the central raphe endings have been little explored as a contributing feature in species delimitation. Mann [10] argued that presence or absence of a central raphe termination is questionable as a character defining species (see also discussion in Trobajo & Cox, [21]), but the deflection of such terminations (when they exist) and the presence of depressions at the valve central nodule have not been clearly studied in culture or from natural populations. The type materials of N. frustulum var. subsalina, N. inconspicua and Nitzschia boliviana show no variation regarding deflection or lack thereof and presence/absence of depressions. Therefore, until shown otherwise, they should be regarded as differentiating features at the species level. Other authors have made similar observations, though not on type material [5] [9] [6] [25], while Trobajo & Cox [21] suggested that further studies are needed to show variability of these features.
The position of velum is one of the least discussed features in relation to genetic or environmentally-related variability. However, a review of the type material of several species of the Section Lanceolatae shows that this feature does not vary at the population level (e.g., Trobajo & Cox [21], Tudesque et al. [24] and Trobajo et al. [23]). Alakananda et al. [1] used this feature in combination with others and argued that they are useful to sepárate species within the Section, which is also the case for the three taxa analyzed here. At the moment, it is unclear if the stability in the position of the velum shown in type materials should be extrapolated to all species within the Section. This is because some morphological variants such as "type 2" found in type material of N. abbreviata Hustedt ex Simonsen and N. invisitata Hustedt exhibit a high variability in pore shape, and probably also in velum features and position, though this has not been shown [22] [23].
The features of girdle bands, except for their closed/open nature, and the occurrence and disposition of perforations, have also been little explored in the literature. Girdle bands of two of the three type populations discussed here were illustrated, and one (N. inconspicua) is shown to have ligulated copulae (fig. 79 in Trobajo et al. [23]), while N. boliviana lacks this structure. Obviously, observations of girdle elements of one or two specimens are insufficient to derive hard conclusions, yet the difference illustrated by Trobajo et al. [23] is worth exploring further.
The shape of fibulae and interfibular spaces have been long used as a feature to distinguish species even under LM. As stated by Denys & Lange-Bertalot [2], extensions of fibulae associated to costae and striae can be useful not only for identification, but also for circumscription of morphologically closely related taxa (see also Alakananda et al. [1]). Especially at the LM level, the shape of the fibulae can be a helpful distinguishing character when used in association with other features such as those discussed here [12] [19] and has even been suggested to indícate evolutionary patterns in certain nitzschioid groups [7]. The distance between median fibulae (the ones flanking the central nodule) have been shown not to vary significantly with environmental conditions and are considered stable characters at the population level [20]. In the type materials presented by Trobajo et al. [23], variability in the shape and distance of the fibulae can be seen at the individual level, but at the population level the variability is different for each of the three taxa in question.
Examination of type material of several infrageneric taxa in Nitzschia, including those in the Section Lanceolatae, showed that valve shape is a reliable character to distinguish taxa at the species level (e.g., Trobajo & Cox [21], Tudesque et al. [24], Hlúbikova [8] and Trobajo et al. [23]). Some variation exists from one individual valve to another, but this is the expected variability found in most diatom populations (Pickett-Heaps et al. [17]). Valve shape in populations of N.frustulum var. subsalina, N. inconspicua and N. boliviana found in type material are rather stable as shown in LM and SEM illustrations presented in Morales & Vis [13] and Trobajo et al. [23], therefore, the comparison shown in Table 1 is reliable and can be used in combination with the other features discussed here to differentiate each of the three discussed taxa.
Culture experiments have shown that valve shape is variable under different conditions in N. frustulum (Kützing) Grunow, and thus, it is an unreliable feature for identification or species boundary delimitation. A more recent molecular study showed that valve outline could not give a clear differentiation of clades or genotypes in the N. inconspicua complex (Rovira et al. [18]; where -as per the analysis implemented here-, an even wider morphological variability than that presented in Trobajo et al. [23] is shown). Here, I show that valve outline can be used in combination with other features to establish discrete morphological variants, which up to this date, and in the absence of clear genetic information, can be considered as different morphological taxa.
Mann [10] warned about the cons of a "sparingly-divided classification". Therefore, until we do not have a clear picture of the limits among taxa at the ecological and molecular level, it is convenient to adopt a splitter approach, especially taking into account that differing morphologies could be indicative of given environmental conditions (i.e., the result of natural selection) and that responses to those conditions might be taxa or even clone-specific [21][18]. Moreover, Rovira et al. [18] argue based rbcL and LSU genetic distances that the N. inconspicua complex could contain several cryptic species. The splitting of apparently cryptic morphologies based on a joint analysis of several qualitative characters, such as the analysis done here, provides a strong hypothesis to be tested in the molecular arena; a hypothesis drawn on morphological characters that go beyond the simple reductionist morphometry.
Acknowledgements
I thank Sinziana Rivera, University of Geneva, for editing the text and reference checking, and Luc Ector, Luxembourg Institute of Science and Technology, for providing literature. I am indebted to Nora Maidana, University of Buenos Aires; Silvia Sala and Amelia Vouilloud, National University of La Plata, Argentina, for reviewing the text, providing valuable opinions and suggesting changes that improved its content.
References
[1] Alakananda, B.; Balasubramanian, K..; Taylor, J.C. & Hamilton, P.B. 2014. Two new species of Nitzschia (Bacillariophyceae) from freshwater environs of Lonar Crater Lake, India. Phycological Research 63:29-36. [ Links ]
[2] Denys, L. & Lange-Bertalot, H. 1998. Observations on two taxa of the section Nitzschiae Lanceolatae (Bacillariophyceae): Nitzschia blankaartensis sp. nov. and N. bulnheimiana. Nova Hedwigia 67: 247-258. [ Links ]
[3] Geissler, U. 1970a. Die Variabilität der Schalenmerkmale bei den Diatomeen. Nova Hedwigia 19: 623-773. [ Links ]
[4] Geissler, U. 1970b. Die Schalenmerkmale der Diatomeen-Ursachen ihrer Variabilität und Bedeutung für die Taxonomie. Beihefte zur Nova Hedwigia 31: 511-535. [ Links ]
[5] Geissler, U. & Gerloff, J. 1963. Elektronenmikroskopische Beiträge zur Phylogenie der Diatomeenraphe. Nova Hedwigia 6: 339-352. [ Links ]
[6] Kobayasi, H. 1985. Ultrastructural differences in certain taxonomically difficult species of Nitzschia section Lanceolatae in Japan. In: Origin and evolution of diversity in plants and plant community (H.Hara, ed.). Academia, Tokyo. pp. 304-315.
[7] Hamilton, P.B. & Laird, K.R. 2001. Nitzschia pseudosinuata sp. nov., a new Holocene diatom from the sediment of Moon Lake, North Dakota, U.S.A. Diatom Research 16: 317-324. [ Links ]
[8] Hlúbikova, D.; Blanco, S.; Falasco, E.; Gomá, J.; Hoffmann, L. & Ector, L. 2009. Nitzschia alicae sp. nov. and N. puriformis sp. nov., new diatoms from European rivers and comparison with the type material of N. sublinearis and N. pura. Journal of Phycology 45: 742-760. [ Links ]
[9] Lange-Bertalot, H. & Simonsen, R. 1978. A taxonomic revision of the Nitzschiae lanceolatae Grunow. 2. European and related extra-European freshwater and brackish water taxa. Bacillaria 1: 11-111. [ Links ]
[10] Mann, D.G. 1982. The use of the central raphe endings as a taxonomic character. Plant Systematics and Evolution 141: 143-152. [ Links ]
[11] Mann D.G. 2010. Discovering diatom species: is a long history of disagreements about species-level taxonomy now at an end? Plant Ecology and Evolution 143: 251–264.
[12] Morales, E.A. & Hamilton, P.B. 2002. Seventh NAWQA taxonomy workshop on harmonization of algal taxonomy. Patrick Center for Environmental Research. The Academy of Natural Sciences of Philadelphia. Report No. 02-21. 34 pp.
[13] Morales E.A. & Vis M.L. 2007. Epilithic diatoms (Bacillariophyceae) from cloud forest and alpine streams in Bolivia, South America. Proceedings of the Academy of Natural Sciences of Philadelphia 156: 123–155.
[14] Morales E.A.; Wetzel C.E.; Rivera S.F.; Van De Vijver B. & Ector L. 2014. Current taxonomic studies on the diatom flora (Bacillariophyceae) of the Bolivian Altiplano, South America, with possible consequences for palaeoecological assessments. Journal of Micropalaeontology 33: 121–129.
[15] Morales E.A.; Wetzel C.E.; Van De Vijver B. & Ector L. 2015. Morphological studies on type material of widely cited araphid diatoms (Bacillariophyta). Phycologia 54: 455-470. [ Links ]
[16] Pickett-Heaps, J. 1983. Valve morphogenesis and the microtubule center in three species of the diatom Nitzschia. Journal of Phycology, 19: 269-281. [ Links ]
[17] Pickett-Heaps, J.; Schmid, A-M.M.; Edgar, L.A. 1990. The cell biology of diatom valve formation. In Progress in Phycological Research, Vol. 7 (Round F.E, & Chapman, D.J., Eds.). Biopress, Ltd. 1-168 pp.
[18] Rovira, L.; Trobajo, R.; Sato, S.; Ibáñez, C. & Mann, D.G. 2015. Genetic and physiological diversity in the diatom Nitzschia inconspicua. Journal of Eukaryotic Microbiology 52: 815-832. [ Links ]
[19] Siver, P.A.; Hamilton, P.B.; Stachura-Suchoples, K. & Kociolek, J.P. 2005. Diatoms of North America. The freshwater flora of Cape Cod. Iconographia Diatomologica 14: 1- 463. [ Links ]
[20] Trobajo, R.; Cox, E.J. & Quintana, X.D. 2004. The effects of some environmental variables on the morphology of Nitzschia frustulum (Bacillariophyta), in relation its use as a bioindicator. Nova Hedwigia 79: 433-445. [ Links ]
[21] Trobajo, R. & Cox, E.J. 2006. Examination of the type material of Nitzschia frustulum, N. palea and N. palea var. debilis. In: Proceedings of the 18th International Diatom Symposium (Ed. by A. Witkowski). Biopress, Bristol. pp. 431-445.
[22] Trobajo, R.; Mann, D.G. & Cox, E.J. 2012. Studies on the type material of Nitzschia abbreviata (Bacillariophyta). Nova Hedwigia, Beiheft 141: 185-199. [ Links ]
[23] Trobajo R.; Rovira L.; Ector L.; Wetzel C.E.; Kelly M. & Mann D.G. 2013. Morphology and identity of some ecologically important small Nitzschia species. Diatom Research 28: 37–59.
[24] Tudesque, L.; Rimet, F. & Ector, L. 2008. A new taxon of the Section Nitzschiae Lanceolatae Grunow: Nitzschia costei sp.nov. compared to N. fonticola Grunow, N. macedonica Hustedt and N. tropica Hustedt and related species. Diatom Research 23: 483-501. [ Links ]
[25] Wendker, S. & Geissler, U. 1988. Investigations on valve morphology of two Nitzschiae lanceolatae. In: Proceedings of the 9th International Diatom Symposium (Ed. by F.E. Round). Biopress, Bristol and Koeltz Scientific Books, Koenigstein. pp. 469–480.