Services on Demand
Journal
Article
Indicators
Related links
Share
Revista Boliviana de Química
On-line version ISSN 0250-5460
Rev. Bol. Quim vol.37 no.3 La Paz Dec. 2020
DOI: 10.34098/2078-3949.37.3.3
FULL ORIGINAL ARTICLES
Manufacture of geopolymeric mortars from ash coming from the Ubinas volcano,
assessment of its mechanical, physical and microstructural properties
Fabricación de morteros Geopoliméricos a partir de ceniza proveniente del volcán Ubinas,
evaluación de sus propiedades mecánicas, físicas y microestructurales
María Vargas1, Marian Hermoza-Gutierrez2, Danny P. Tupayachy-Quispe1,
Jonathan Almirón2, Paul K. Huanca Z.3,* , Francisco J. Velasco-López4
1 Laboratorio de Ciencia de los Materiales. Facultad de Ciencias e Ingenierías Físicas y Formales,
Universidad Católica de Santa María, Samuel Velarde 320, Arequipa, Perú, www.ucsm.edu.pe
2Escuela Profesional de Ingeniería Ambiental, Facultad de Ingeniería de Procesos FIP,
Universidad Nacional San Agustín de Arequipa UNSA, Av. Independencia s/n-Pab.
Antonio Raimondi-4to piso, Área de Ingenierías, phone +5154201723, Arequipa, Perú,
ambiental@unsa.edu.pe, http://fip.unsa.edu.pe/ingambiental/
3Escuela Profesional de Ingeniería de Materiales, Facultad de Ingeniería de Procesos FIP,
Universidad Nacional San Agustín de Arequipa UNSA, Av. Independencia s/n-Pab.
Ing. Materiales, phone +5154200037, Arequipa, Perú,
materiales@unsa.edu.pe, http://fip.unsa.edu.pe/ingmateriales/
*Correspond¡ng author: phuanca@unsa.edu.pe
4Materials Science and Engineering Department-IAAB, University Carlos III of Madrid, Avda.
Universidad N° 30, 28911 Leganés, Madrid, Spain. www.uc3m.es o
Received 06 02 2020 Accepted 08 04 2020 Published 08 30 2020
Abstract
In this study, it has been proposed a geopolymerization method that uses a low liquid/solid (L/S) ratio and the pistón as a compaction method which has favored the compression strength achieved considering that the synthesis of geopolymers is characterized by the use of an alkaline solution with a high L/S ratio (greater than 0.45).
The volcanic ashes from the Ubinas volcano (Peru's most active volcano) that are rich in Al2O3, SiO2 and CaO were alkaline activated with sodium hydroxide (NaOH) and sodium hydroxide with addition of sodium silicate (NaOH+Na2SiO3), both solutions with 12 M concentration, with a low liquid/solid (L/S) ratio of 0.1 and solid:solid (S:S) ratio of 1:1. Volcanic ashes and Portland cement were used as initiators and the local sand was used as aggregate for both of them.
The mechanical, physical (density and water absorption) and structural properties were determined by X-ray diffraction and the microstructure was determined by scanning electrón microscope from the mortars. As a result, it has been proven that the use of the NaOH solution favors the dissolution of alumina and silica and therefore the polycondensation, mainly when using an initiator with a high composition of AI2O3, SiCh and CaO. Besides, it has been demonstrated that the excess of NaOH (using the sodium silicate solution) inhibits the polycondensations. Therefore, this method has the advantage of a lower use of L/S ratio than the other methods. It has been obtained a compressive strength of 24.6 ± 1.7 MPa with the geopolymer mortars activated with NaOH and 14.2 ± 2.3 MPa with the geopolymer mortars activated with NaOH+Na2SiO after 28 days of curing being these valúes higher than those for mortars in Norm NTP 334.051.
Keywords. Volcanic ash, Geopolymer, Concrete, Compressive strength.
Resumen
En este estudio, se ha propuesto un método de geopolimerización que utiliza una baja relación líquido/sólido (L/S) y el pistón como método de compactación, lo que ha favorecido la resistencia a la compresión lograda, teniendo en cuenta que la síntesis de geopolímeros se caracteriza por el uso de una solución alcalina con una alta relación L/S (mayor que 0.45).
Para la síntesis de los morteros se usaron cenizas provenientes del volcán Ubinas (el volcán más activo del Perú), que son ricas en AI2O3, SiO2 y CaO, las cuales fueron activadas alcalinamente con hidróxido de sodio (NaOH) e hidróxido de sodio con la adición de silicato de sodio (NaOH+Na2SiO3), ambas soluciones en una concentración de 12 M, con una baja relación líquido/sólido (L/S) de 0.1 y una relación sólido:sólido (S:S) de 1:1. Las cenizas volcánicas y el cemento Portland se usaron como iniciadores mientras que la arena local se usó como agregado para ambos.
Las propiedades mecánicas, físicas (densidad y absorción de agua) y estructurales de los morteros se determinaron por difracción de rayos X y la microestructura se determinó a través del microscopio electrónico de barrido.
Como resultado, se ha demostrado que el uso de la solución de NaOH favorece la disolución de alúmina y sílice y, por lo tanto, a la policondensación. Esto principalmente cuando se usa un iniciador con una alta composición de AI2O3, SiO2 y CaO. Además, se observó que el exceso de NaOH (usando la solución de silicato de sodio) inhibe las policondensaciones.
Por lo tanto, este método tiene la ventaja del uso de una relación L/S baja en comparación con las otras relaciones L:S que suelen ser altas. Se ha obtenido una resistencia a la compresión de 24.6 ±1.7 MPa con los morteros de geopolímero activados con NaOH ydel4.2±2.3 MPa con los morteros de geopolímero activados con NaOH+Na2SiO3 después de 28 días de curado, siendo estos valores mayores que los indicados para morteros según la Norma Técnica Peruana (NTP) 334.051.
Palabras clave. Ceniza volcánica, Geopolímero, Hormigón, Resistencia a la compresión.
INTRODUCTION
The current problem that increasingly affects the physical, chemical and biological components with whom the living beings interact is a consequence of environmental pollution. This is mainly about the inadequate industrial methods that lead to the overexploitation of the natural resources and breed hazardous waste during the production processes. One of the main challenges to face the environmental pollution comes from the construction industry, which is responsible for a significant proporción of the environmental contamination, due to the production of cement (concrete), since the world needs this material [1].
The production of Ordinary Portland Cement (OPC) is an intensive energetic process that requires large quantities of fuel combustión and decomposition of limestone. This results in extreme emissions of carbón dioxide, being around 6% to 7% from the worldwide emission valúes and it is expected to increase in 200% by 2050 [2].
Among the technologies which are considered to reduce and mitígate the emissions of CO2 produced by cement production, there is one that applies the replacement of raw material throughout the development of new sustainable and environmentally friendly materials called geopolymers [3]. One of the main advantages of the development of geopolymers is that raw materials can be by-products of low-cost industrial wastes (fly ash, silica fume, slag, rice husk ash, red mud, etc.), with high silicon (Si) and aluminum (Al) contents, or natural sources composed of aluminosilicates.
The inorganic polymer concretes (IPC), also called Geopolymer concretes (GC), alkali cement or geocement are emergent materials with different applications that come from waste management into the construction industry, while the pozzolanic cements rely on the presence of calcium [4]. The IPCs do not present calcium-silica-hydrated (C-S-H) gels forming the matrix, but polycondensations of initiators high in AI2O3, SiCh and alkalis to achieve structural strength [5,6]. Generally, the geopolymer mortars (GM) are reported to be more sustainable than OPC on account of the low energy required for their production with significantly lower emissions of CO2, good physical and mechanical properties, and durability [7,8]. Therefore, GM based on volcanic ashes have been synthetized at 27°C and 80°C with a mixed solution of sodium silicate and sodium hydroxide, where it was observed that the permeability, porous structure and curing temperature are key factors for the durability of GM. Those GM can achieve a compressive strength of 37.9 MPa for the specimens cured at 80°C, and 20.5 MPa for the specimens cured at 27oC for 90 days [8].
Additionally, GMs based on volcanic ashes, metakaolin, and river sand have been synthetized with an alkali solution of sodium silicate and sodium hydroxide. The addition of metakaolin consumes the excess of sodium hydroxide and allows the dissolution of silica and alumina present in the volcanic ash resulting in an improvement of the compressive strength of mortar up to valúes around 68.8 MPa [9]. It has also been determined that the different characteristics in the chemical composition of volcanic ash collected from different zones influence the properties of synthetized geopolymers, resulting that the volcanic ash with lower SiO2/AI2O3 ratio (about 4.55, red color) promote higher compressive strengths of 50 MPa as well as grey color ashes, with a SiO2/AI2O3 ratio of 4.90, promote a compressive strength of 23 MPa [10], [11]. These studies were carried out to use the volcanic ashes as a raw material to obtain GMs considering the abundance of such material in some places.
Peru is the sixth country in Latin-America to produce cement behind México, Brazil, Argentina, Colombia and Venezuela [12]. Because of the demand of cement industry in the national trade, studies come up to replace the OPC by using volcanic ashes, an available material in nature due to the large chain of volcanoes placed in the south of Pera. This área comprises the northern part of the Volcanic Central Zone (VCZ) in South America. Therefore, in Pera, 400 volcanoes have been identified, being most of them inactive. In the last decade, it has been identified activities in some volcanoes that are located in Arequipa (Misti and Sabancaya), Moquegua (Ubinas, Huaynaputina and Ticsani) and Tacna (Tutupaca and Yucamane) departments, which have historical volcanic eraptions [13]. The Ubinas Volcano is considered the most active volcano in the south of Pera due to its 25 events of fumarolic activities with fly ash and rocks since the 16th century, being the last volcanic eraption in 2019 according to INGEMMET.
Considering that volcanic ash is a raw material due to its composition based on aluminosilicates, this study focuses on the evaluation of the mechanical behavior, physical and microstractural properties of synthetized geopolymer mortars at a lower concentration of activating solution than the usual, resulting in decreasing the curing time and a compressive method that allows the cost to be low. Additionally, the specimens were compared to the Portland cement mortars synthetized by the same compressive method.
EXPERIMENTAL
Materials
The raw materials used as initiators in the preparation of mortars were the Multipurpose Cement of High Durability type IP from YURA (YC) and volcanic ashes from the Ubinas Volcano (VA). Local sand (LS) from SUPERMIX was used as fine aggregate. The materials were dried in a stove at 110 + 5 °C for 24 h to avoid inconveniences on the results due to the atmospheric moisture [14].
Characterization of initiators and aggregates
For the characterization of raw materials (initiators) and aggregates, the chemical composition was determined by X-ray fluorescence (XRF) with a Spectro Xepos (Ametek Materials Analysis División) equipment.
The thermal properties were evaluated by the thermogravimetric analysis (TGA) using a STA 6000 Perkin Simultaneous Thermal Analyser with a set temperatura from 50°C until 900°C and a Polyscience cooling system with N2 gas with a flow of 20 ml/min.
Finally, the mineralogical phases were determined by X-ray diífraction tests using a Bruker diífractometer, model D8 Avance Davinci, with CuKα radiation (λ = 0.1542 nm), voltage of 40 kV and current of 40 mA. The materials were evaluated at 2θ between 10° and 80°, and at a sean speed of 27min.
Synthesis of mortars
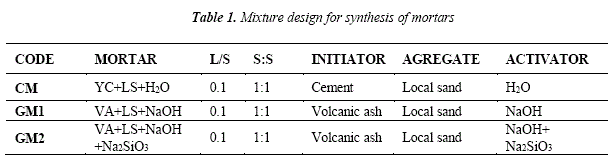
The methodology employed for the synthesis of geopolymer specimens included automated mixing, compression in pistón mold and curing at laboratory-controlled conditions. The geopolymer and cement mortars were produced with asolid:solid (S:S) ratio of 1:1, concerningto cement:sand or fly ash:sand, and a liquid-solid (L/S) ratio of 0.1.
For the production of specimens, the volcanic ash from Ubinas Volcano (VA) and cement as raw materials, and local sand (LS) as aggregate were weighed. The activating solutions were 12 M NaOH and 12 M sodium hydroxide with addition of sodium silicate (NaOH+Na2SiO3) with a proporción of 75%-25% for each one [15,16]. Table I shows the mixture design.
The raw materials and aggregate were mixed with the activating solution in an automatic mixer Yu Feng model Cement Mortar Mixer JJ-5. Subsequently, the mixture was splitted off in three parts into the pistón mold to be rammed by rods under a pressure of 15 MPa in a Universal Compression machine Kinsgco model KJ-106662.
Finally, the specimens were removed to register the weight, and covered with plástic to avoid the loss of moisture by evaporation in the oven. The starting curing was at 80°C for 48 h in a universal oven Memmert model UN55. The specimens were left in a Universal Incubator Memmert model IF 110 at room temperature and monitored moisture.
Characterization of mortars
The physical properties such as bulk density and real density were determined by the gravimetric method (according to ASTM Standard C-20). The water absorption was also determined by the Archimedes method (according to ASTM C-642 "Standard Test Method for Density, Absorption, and Voids in Hardened Concrete") after 28 days of curing.
The mechanical characterization of mortars was made throughout compressive tests according to the "ASTM Standard Test Method C39: Compressive Strength of Concrete Cylinders" [17] in a Compressive Hydraulic Machine ELE International after 7, 14 and 28 of curing days.
The microstructural properties were determined by X-ray diffraction (XRD) in a diffractometer Bruker D8 Advance Davinci (Model FQ5060F) with radiation of Cu Ka, where the diffraction patrons were collected at 29 from 10° to 80° with a sean speed of 2°/60 s. A scanning electrón microscope (SEM) Hitachi (Model SU8230) with back-scattered electrón (BSE) detector was used in order to complement the microstructural analysis of the phases in mortars. Additionally, the semiquantitative chemical composition in mortars was determined by the analyzer EDAX, energy-dispersive X-ray spectroscopy (EDX). Finally, the microphotographs were taken at 5 kX.
RESULTS AND DISCUSSION
Characterization of raw materials
Chemical composition
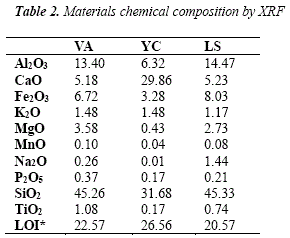
The chemical compositions of raw materials (initiators) determined by XRF are shown in Table 2. The volcanic ash shows significant percentages of SiO2, AI2O3 and Fe2O3/ with SiO2/AI2O3 molar ratio of 5.73. This valué is cióse to the ratio used to synthesize geopolymers from volcanic ashes, rangingbetween 4.55 and 4.90 [10].
In [8] it was reported similar valúes of SiO2 and AI2O3 in volcanic ashes (46.28% and 15.41% respectively); however, there are differences in valúes for Fe2O3 (13.32%), CaO (9.07%), MgO (6.74%) and Na2O (3.88%). This volcanic ash was used for the production of geopolymer mortars where it was found that alumina is relevan: for the efflorescence mitigation.
The hydraulic cement is composed of a variety of elements. primarily as CaO due to the lime, SiO2, AI2O3 and Fe2O3 due to the clay. The local fine sand has a high amount of SiO2, followed by AI2O3, Fe2O3, CaO and K2O which contribute in favorable chemical properties to interact with binders (linkers) as in [20].
X-ray diffraction
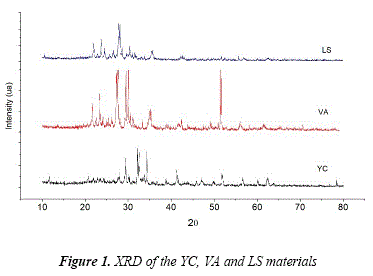
The XRD analysis of YC and VA initiators and LS aggregate are shown in Figure 1. The YC diffractogram shows the presence of minerals such as tricalcium silicate known as alite (C3S, PDF: 86:38-1429) with peaks 2θ at 29.35°, 34.35° and 41.29°, dicalcium silicate known as belite (C2S, PDF: 70-0388) with peaks 26 at 32.62° and 34.41°, plaster (PDF: 03-0044) with peaks 2θ at 11.64° and 20.73° and tricalcium aluminate known as celite (C3A, PDF: 38-1429) with peaks 26 at 47.62° as in [21]. All of them are generally created during the production of clinker, especially during the sintering phase (heat treatment at lower temperature than the melting point) where the main elements represent the 95% of cement.
The bands present in the volcanic ash diffractogram denote the presence of compounds with high aluminum and silicon contents, and also minerals composed by calcium, sodium, potassium and some impurities high in Fe and Ti. The mineralogical composition of VA shows feldspar (Na (CaAlSi3O8), PDF:89-8575) and anorthite (Ca (Al2Si2O8), PDF: 89-1459) as principal minerals based on the intensity of their main three peaks which are the highest in comparison with the other minerals. Other minerals were identified such as aluminum diopsides ([Ca(Mg,Fe,Al)(Si,Al)2O6], PDF: 38-0466), augite (Ca0.61Mg0.76Fe0.49(SiO3)2, PDF:76-0544), forsterite (Mg2Si04, PDF:85-1357), diopside ([(Ca0.52Na0.29Fe0.10Mg0.09)(Mg0.057Fe0.14Al0.27Mn0.01Ti0.01)(Si206)], PDF: 85-1692) and hematite (Fe2O3, PDF #03- 0812).
DRX analysis of fine aggregate shows a composition high in silicon and aluminum regarding feldspar and anorthite with a 26 range of 20° - 30°.
Thermal analysis
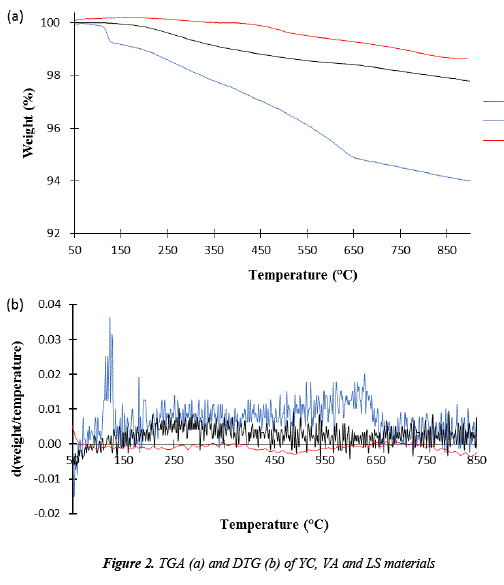
TGA and dTGA thermograms are shown in Figure 2. The TGA thermogram of Cement (YC) indicates that a weight loss takes place at 100 °C, probably related to the evaporation of water in calcium sulfate dihydrate or plaster as in [21]. Also, there is a peak at 660 °C, in the range of 600 and 750°C, that belongs to decarbonated limestone which comes from vaterite.
The VA thermogravimetric curve shows the lowest weight loss in comparison to LS and YC, being the residual mass of 98.6%. The curve of the first derivative shows stability concerning the speed in weight loss with increasing temperatures. Ref. [22] indicates that the wave from 450 a 650 °C can derive from the decomposition of kaolinite at low proportions. The experimental thermogravimetric result for local sand (LS) shows that there was a weight loss of 2.2%, related to a dehydration process between 100 and 200 °C.
Characterization of Mortars
Physical properties
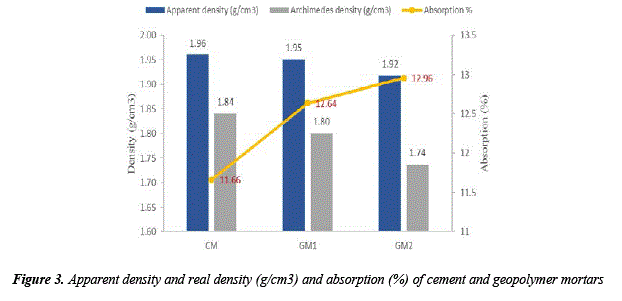
The strongest and probably the most impermeable mortar will be the one who has the highest density, in other words, the one whose volume contains the highest percentage of solid materials [23]. In Figure 3, it is shown that the two groups of geopolymer mortars obtained from the alkali activation of volcanic ash absorb more water than the cement mortars.
Generally, the absorption of water is related to apparent porosity (PA) [8]. Both properties are related to the presence of open and cióse pores on mortar samples. This presence is due to water reléase that comes from water that does not react during the geopolymerization [8]. Therefore, according to the results of water absorption, it can be inferred for geopolymer mortars that the geopolymerization was higher in mortars that were alkali activated with NaOH only; on the contrary, the geopolymerization was lower in mortars that were alkali activated with NaOH and sodium silicate.
It is important to indicate that the water absorption is 5.91% for geopolymer mortars synthetized from volcanic ash with NaOH and sodium silicate (L/S: 0.45) at 80 °C with a curing time of 24 h. The water absorption for geopolymer mortars synthetized in this study was 12.96%. Therefore, the L:S ratio and the curing time influence on water absorption, apparent porosity properties and density.
Figure 3 shows the density of mortars revealing the inverse proportion concerning the absorption results (related to porosity). In [8] also it was reported this behavior, being an inverse proportional relationship between density and water absorption in mortars, while there is a direct proportional relationship between apparent porosity and water absorption. In this case, CM has the highest density, while GM2 sample has the lowest density but the highest water absorption this because of the water loss that did not react during the geopolymerization causing the sample to be more porous and lighter with time as in [8].
However, in [24], the porosity can be favorable due to the use of bricks with low density and high porosity for the production of domes in construction of historical buildings in Anatolia. Those bricks have densities of 1.7-1.8 g/cm3, and porosities of 33-37% [24]. In this case, mortars have higher density, therefore they can be used in the partition industry.
Mechanical properties
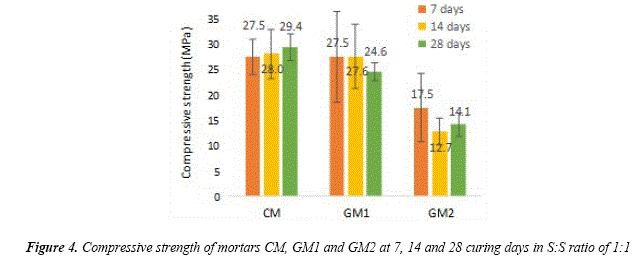
Figure 4 shows the compressive strength of cement mortars and geopolymer mortars based on volcanic ash with S:S ratio of 1:1. Forthe S:S ratio of 1:1, it is shownthat the strength of geopolymer mortars based on volcanic ashes has a similar mechanical behavior to the cement mortars (29.4 MPa) with a slight decrease to 24.6 MPa on the compressive strength for mortars synthetized with NaOH after 28 days of curing. On the other side, mortars based on volcanic ashes alkali activated with NaOH and Na2SiO3 (75% - 25%) have a significant decrease of 14.2 MPa on the compressive strength after 28 days of curing. In [8,25] it has been observed that the mechanical strength depends on the physical properties mentioned before, so a high strength mortar will correspond to lower porosity and water absorption while a low strength mortar will be lighter, with higher porosity and water absorption. In [26] it is also reported this behavior in the compressive strength of mortars that have been synthetized with NaOH and sodium silicate due to the chemical interaction between the raw materials being with the sodium silicate, the one that has the lowest compressive strength.
According to [10], volcanic ashes with high amount of AI2O3, SiO2 and CaO in an amorphous phase have better compressive strength. This can explain the high compressive strength valúes in mortars based on volcanic ashes with NaOH in comparison to cement mortars. However, the aluminosilicates must react with the alkali solution so silica and alumina can dissolve. The dissolution of silica and alumina improves the polycondensation and the development of polymeric chains that increase the compressive strength in geopolymer mortars. Additionally, an excess of NaOH and sodium silicate inhibits the polycondensations, so it explains why the reaction with these activators do not contribute to the compressive strength [9].
The compressive strength in polymers relies on the resistance between the geopolymer gel and aggregate. The results suggest a negative variation of compressive strength between geopolymer mortars with NaOH and those developed with NaOH and sodium silicate due to the chemical interaction in materials that constitute mortars [26]. This mechanical behavior could be caused by the composition of the sand and the high amount of silica (70.72% SiO2) in the aggregate which avoids the full reaction of the activating solution. Additionally, it is well known that CaO has a significant influence in the properties of geopolymer cements due to its abundantly presence in CaO composition as in [10].
It is important to mention that, in this research, a L/S ratio of 0.1 was used, obtaining a high compressive strength for sample GM1 with a valué of 24.59 MPa, which is similar to the valúes of samples based on volcanic ashes of 30 MPa and 20.5 MPa (curing time of 28 and 27 days at 80°C and 27°C, respectively) with a L/S ratio of 0.45 [8]. Additionally, another important factor to get high compressive strength is the surface área [10]. The volcanic ash from Ubinas Volcano has a low surface área of 2.06 m2/g as in [19], but despite this, high compressive valúes in samples with a low molar activating solution were reached. In [27] they prepared a cement mortar based on volcanic ash in a relation of 1:1 which got a compressive strength of 16.5 MPa at 28 days of curing time, this valué decreased at a higher amount of volcanic ash. This prove that the activation of volcanic ash with an alkali solution improve the compressive strength. It is important to mention that these valúes obtained are over those for mortars in NormNTP 334.051.
Microstructural Properties
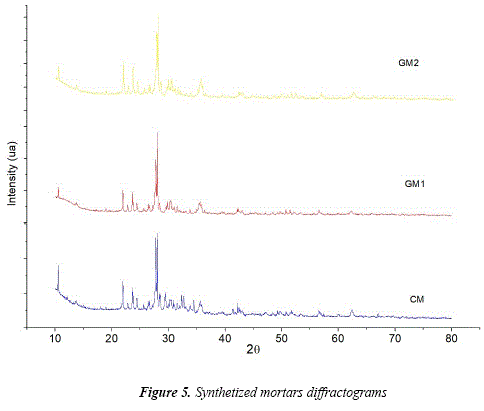
Figure 5 shows the diffractograms of mortars. The presence of hydrated cements of Portland cement is easy to identify by XRD. There are 29 peaks of the main hydrated elements of Portland cement paste at 34.1° and 18.1°. In [28] they refer to the presence of Ca(0H)2 or portlandite in cement mortars and the presence of CaCO3 in hydrated cement. There are three crystal polymorphic forms how the CaCO3 can be found in carbonate Portland cement concrete: calcite (limestone), aragonite and vaterite. The crystal form in which it appears relies on the environmental conditions that it is exposed (humidity-moisture) [28]. [29] mentions that CaCO3 in Portland cement with high humidity (>90%) will be as aragonite. In addition, the formation of vaterite and aragonite in Portland cement concrete can take place simultaneously by the absorption of CO2 from the environment. According to the diffraction patterns, the crystal form of vaterite is located in 29 peaks at 27.1°, 32.8° and 50.1° as in [28].
The patterns of synthetized geopolymer mortars show the same crystal phases as volcanic ash. The main changes relate to the intensity of peaks of crystal phases located between 21° and 38° (29), which are also located inside the halo between 18° and 40° of the geopolymers amorphous phase as in [10]. The GM1 geopolymer mortar is predominantly amorphous causing the presence of mineralogical structures such as andesine, augite and anorthite in a minor intensity to the raw material.
The geopolymer mortar GM2 shows peaks at lower intensity than GM1, causing the presence of mineralogical structures such as andesine, silicon oxide and diopside. This indicates that there is an effect in the dissolution of volcanic ashes, aggregate and the alkali activator with sodium silicate that influences in the reactivity of the material, thus affecting the mechanical (compressive strength) and physical (absorption and density) properties of the sample. The drecrease of the amorphus phase after the geopolymerization could be related to the pardal recrystalization.
Although there was not a new peak after the geopolymerization, it must be considered that the pardal crystalization of the amorphous phase in minerals as the zeolite can occur at temperatures that are used in the geopolymer synthesis, specially when the NaOH is used as an activator [30]. According to [31], the presence of soluble silicate in the activator difficults the reorganization of the local structures of geopolymer gels and also inhibit the formation of zeolites or predecesors (initiatios) of zeolites. Therefore, it is probable that such minerals are present in the cured sample, but in a minor quantity or below the detection limit of the equipment.
Mortars Morphology
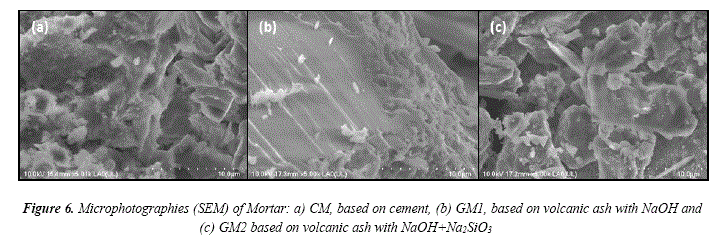
SEM image of mortars are shown in Figure 6. The plañe and continuous surfaces are shown in Figure 6a, as well as, the presence of discontinuous porous and clean zones that belong to the sand and cement.
The microphotograph of GM1 mortar is shown in Figure 6b, where the sample shows a dense microstructure with a minor presence of micropores. There is a bonding between the aggregate and the geopolymer binder and also there were no cracks in the interface, so there is a strong bonding. A thorough inspection in the interfacial región suggests that a pardal dissolution could take place in the aggregate surface causing a new interface. In this specimen, it is observed that the geopolymer mortars were produced by the pardal dissolution of the sand. The presence of micropores may belong to the material that has not reacted.
When the alkali activators are added, most of the particles get dissolved while others do not. The dissolved partióles form a matrix that contains the reaction producís. When the material hardens, the reaction continúes on the surface of particles that did not dissolve, creating a solid reaction ring around them. These rings indicate that after the material has hardened the activators keep attacking the particles surface and the geopolymerization reaction keeps going on [32]. It is posible that the aggregate that is not linked to the activator may be still in the reaction process.
Figure 6c shows that GM2 sample does not have an homogeneus matrix and there are particles of ashes and aggregates. Also, it shows needle shaped crystal structures which could be related to aluminosilicates that did not react and are negatively influencing the compressive strength [30].
These results fit with the compressive strength results, where the GM1 sample has higher compressive strength, a denser microstructure and a minor quantity of micropores than GM2 sample.
CONCLUSIONS
Geopolymer mortars were synthetized from volcanic ash and fine local aggregate, and alkali-activated with sodium hydroxide (NaOH) and sodium hydroxide with sodium silicate (NaOH+Na2SiO3) in an L/S ratio of 0.1 under the pistón mold compressive method at 80 °C and 48 h of curing. Despite the low L/S ratio, high compressive strengths for geopolymer mortars with NaOH (GM1) were reached with valúes of 27.5 MPa, 27.6 MPa and 24.6 MPa for samples with 7, 14 and 28 of curing days, respectively. These valúes are over those for mortars in Norm NTP 334.051. The geopolymer mortars alkali activated with NaOH and sodium silicate (GM2) had strengths of 12.7 MPa, 14.2 MPa and 17.5 MPa for samples with 7, 14 and 28 of curing days, respectively. The data were verified using the proposed method for cement mortars getting strength valúes of 27.5 MPa, 28.0 MPa and 29.4 MPa with 7, 14 and 28 curing days, respectively. As a result, the proposed method is effective for GM1.
The physical properties of mortars correlated with the compressive strengths. GM2 sample had higher absorption (12.96%) with a lower compressive strength followed by GM1 sample (12.64%) and cement mortar (11.66%). This was also observed in the morphology, the GM2 sample had a homogeneous microstructure with crystal structure that have not reacted in comparison to the GM1 sample that had a dense microstructure.
The GM1 mortars had mineralogical structures type andesine, augite and anorthite with a homogeneous matrix which suggests a strong connection between the reacted components. For the GM2 mortars, the mineralogical structures such as andesine, silicon oxide and diopside were identified.
ACKNOWLEDGMENT
The authors are gratefiil to the Materials Science and Engineering and Chemical Engineering Laboratory from Carlos El University of Madrid. National Council of Science, Technology and Technological Innovation of Pera.
REFERENCES
1. Huseien, G.F., Mirza, J., Ismail, M., Ghoshal, S.K., Hussein, A.A. 2017, Geopolymer mortars as sustainable repair material: A comprehensive review, Renew Sustain Energy Rev, 80, 54-74. DOI: 10.1016/j.rser.2017.05.076 [ Links ]
2. Shi, C, Fernández-Jiménez A., Palomo, A. 2011, New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem ConcrRes, 41(1), 750-63. DOI: https://doi.org/10.1016/i.cemconres.2011.03.016 [ Links ]
3. Xie, T., Ozbakkaloglu, T. 2015, Behavior of low-calcium fly and bottom ash-based geopolymer concrete cured at ambient temperature, Ceram Int, 41(4), 5945-5958. DOI: https://doi.org/10.1016/i.ceramint.2015.01.031 [ Links ]
4. Phair, J.W., Van Deventer, J.S.J., Smith, J.D. 2004, Effect of Al source and alkali activation on Pb and Cu immobilisation in fly-ash based geopolymers, Appl Geochemistry, 19(3), 423-434. DOI: https://doi.org/10.1016/S0883-2927(03)00151-3 [ Links ]
5. Yip, C.K.B. 2014, The role of calcium in geopolymerisation, PhD thesis, The University ofMelbourne, Melbourne, Australia, retrieved from: https://minerva-access.unimelb.edu.au/handle/l 1343/39281. [ Links ]
6. Sofi, M., Van Deventer, J.S.J., Mendis, P.A. Lukey, G.C. 2007, Bond performance of reinforcing bars in inorganic polymer concrete (WC), JMater Sa'42, 3107-3116. DOI: https://doi.org/10.1007/sl0853-006-0534-5 [ Links ]
7. Duxson, P., Provis, J.L., Lukey, G.C, Van Deventer, J.S.J. 2007, The role of inorganic polymer technology in the development of green concrete, Cem ConcrRes, 37(12), 1590-1597. DOI: https://doi.org/10.1016/i.cemconres.2007.08.018 [ Links ]
8. Djobo-Yanka, J.N., Elimbi, A., Tchakouté-Kouamo, H., Kumar, S. 2016, Mechanical properties and durability of volcanic ash based geopolymer mortars, Constr BuildMater, 124, 606-614. DOI: 10.1016/i.conbuildmat.201 [ Links ]
9. Tchakoute-Kouamo, H., Mbey, J.A., Elimbi, A., Kenne-Diffo, B.B., Njopwouo, D. 2013, Synthesis of volcanic ash-based geopolymer mortars by fusión method: Effects of adding metakaolin to fused volcanic ash, Ceramics International, 39(2), 1613-1621. DOI: https://doi.org/10.1016/i.ceramint.2012.08.003 [ Links ]
10. Tchakoute-Kouamo, H.,, Elimbi, A., Yanne, E., Djangang, C.N. 2013, Utilization of volcanic ashes for the production of geopolymers cured at ambient temperature, Cement and Concrete Composites, 38, 75-81. DOI: https://doi.org/10.1016/i.cemconcomp.2013.03.010 [ Links ]
11. Djobo-Yanka, J.N., Elimbi, A., Tchakouté-Kouamo, H., Kumar, S. 2017, Volcanic ash-based geopolymer cements/concretes: the current state of the art and perspectives, Environ Sci PollutRes, 24(5), 4433-4446. DOI: https://doi.org/10.1007/sl 1356-016-8230-8 [ Links ]
12. Saavedra-Vera, J.V. 2011, Cemento Portland. https://vdocuments.es/-cemento-portlanddocx.html. Access date: May 2020. [ Links ]
13. Macedo-Sánchez, O.E. 2013, Investigación sobre volcanes activos en el sur del Perú: Reporte Técnico Especial. https://repositorio.igp.gob.pe/handle/IGP/1217. Access date: May 2020. [ Links ]
14. NTP 339.127. Norma Técnica Peruana: Método de ensayo para determinar el contenido de humedad de un suelo. 1999, 12. https://es.scribd.com/doc/316683627/ntp-339-127-suelos-metodo-de-ensavo-para-determinar-el-contenido-de-humedad-de-un-suelo-ntp-pdf. Access date: May 2020. [ Links ]
15. Camacho-Torres, J.J. 2018, Estudio técnico y económico de un nuevo material para uso potencial en construcción, obtenido a partir de ceniza del Volcán Ubinas, BSc dissertation, Universidad Católica San Pablo, Arequipa, Perú, retrieved from: https://core.ac.uk/download/pdf/225490094.pdf. [ Links ]
16. Pérez Huamaní, L.C. 2018, Evaluación mecánica y química de geopolímeros obtenidos a partir de ceniza del volcán Ubinas activada con hidróxido de sodio y silicato de sodio para determinar su desempeño conforme a normativa para un adocreto. BSc dissertation, Universidad Católica San Pablo, Arequipa, Perú, retrieved from: http://repositorio.ucsp.edu.pe/handle/UCSP/15708. [ Links ]
17. Anónimo. 2020, C39/C39M-18 A Método de Ensayo Normalizado para Resistencia a la Compresión de Especímenes Cilindricos de Concreto. https://www.inteco.org/shop/product/inte-c39-2006-metodo-de-ensavo-para-la-resistencia-a-la-compresion-uniaxial-de-especimenes-cilindricos-de-concreto-2256?variant=7620. Access date May 2020 [ Links ]
18. Part, W.K., Ramli, M., Cheah, C.B. An Overview on the Influence of Various Factors on the Properties of Geopolymer Concrete DerivedFrom Industrial Byproducts, In: Handbook ofLow Carbón Concrete, ed by Nazari, A., Sanjayan, J., 1 st edition, 2017, Elsevier Science, B.V., Amsterdam, Holland. DOI: doi.org/10.1016/J.CONBUILDMAT.2014.12.065. [ Links ]
19. Almirón, J., Roudet, F., Duquesne, S. 2019, Influence of volcanic ash, rice husk ash, and solid residue of catalytic pyrolysis on the flame-retardant properties of polypropylene composites, Journal of Fire Sciences, 37(4-6), 434-151. DOI: https://doi.org/10.1177/0734904119867912 [ Links ]
20. Subaer, S.. 2004, Influence of aggregate on the microstructure of geopolymer, PhD thesis, Curtin University of Technology, Perth, Australia, retrieved from: http://hdl.handle.net/20.500.11937/1695 [ Links ]
21. Flores-Ledesma, A., Barceló-Santana, F.H., Bucio-Galindo, L., Arena-Alatorre, J.A., Ruvalcaba-Sil, J.L. 2016, Elemental chemical composition and phase analysis by means of PKE, DSC, TGA, and DRX of MTA Ángelus® and a white Portland cement, Revista Odontológica Mexicana, 20(3), el82-el86. DOI: https://doi.org/10.1016/i.rodmex.2016.08.015 [ Links ]
22. Serra, M.F., Conconi, M.S., Suarez, G., Aglietti, E.F., Rendtorff, N.M. 2015, Volcanic ash as flux in clay based triaxial ceramic materials, effect of the fíring temperature in phases and mechanical properties, Ceram Int., 6(5), 6169-6177. DOI: 10.1016/i.ceramint.2014.12.123 [ Links ]
23. Gutiérrez de López, L. 2003, El concreto y otros materiales para la construcción., BSc dissertation, Universidad Nacional de Colombia, Manizales, Colombia, retrieved from: https://repositorio.unal.edu.co/handle/unal/9302 [ Links ]
24. Ugurlu Sagin, E., Bóke, H. 2013, Characteristics of bricks used in the domes of some historie bath buildings, Journal of Cultural Heritage. 14S(3), e73-e76. DOI: http://dx.doi.Org/10.1016/i.culher.2012.ll.030 [ Links ]
25. Galvez-Escalante, L.J. 2018, Influencia de los huesos calcinados por arena, módulo de finura y relación cemento: arena sobre la resistencia a la compresión, densidad y capilaridad durante la elaboración de morteros modificados, BSc dissertation, Universidad Nacional de Trujillo, Trujillo, Perú, retreived from: http://dspace.unitru.edu.pe/handle/UNITRU/10636 [ Links ]
26. Temuujin, J., Van Riessen, A., Mackenzie, KJ.D. 2010, Preparation and characterisation of fly ash based geopolymer mortars, Constr BuildMater, 24(10), 1906-1910. DOI: https://doi.org/10.1016/i.conbuildmat.2010.04.012 [ Links ]
27. Siddique, R. 2011, Effect of volcanic ash on the properties of cement paste and mortar, Resources, Conservation and Recycling, 56, 66-70. DOI: 10.1016/j.resconrec.2011.09.005 [ Links ]
28. Blanco, M.T., Puertas, F.,, Vázques T., De la Fuente, A. 1992, Técnicas y métodos más adecuados para la identificación del cemento aluminoso y de cemento de base portland en hormigones, Materiales de Construcción, 42(228), 51-64. DOI: 10.3989/mc. 1992.v42.i228.696 [ Links ]
29. Takemoto, K. , Saiki, Y. 1962, Infrared absorption spectra of dihydrate, hemihydrate, soluble anhydrite and insoluble anhydrite of calcium sulfate, Gypsum Lime, 61, 277-283. DOI: https://doi.org/10.1145l/mukimatel953.1962.277 [ Links ]
30. Lemougna, P.N., Chinje-Melo, U.F., Delplancke, M.P., Rahier, H. 2014, Influence of the chemical and mineralogical composition on the reactivity of volcanic ashes during alkali activation, Ceram Int 40(1), 811-820. DOI: http://dx.doi.Org/10.1016/i.ceramint.2013.06.072 [ Links ]
31. Zhang, Z., Provis, J.L., Wang, H., Bullen, F., Reid, A.. 2013, Quantitative kinetic and structural analysis of geopolymers. Part 2. Thermodynamics of sodium silicate activation of metakaolin, Thermochimica acta, 565, 163-171, DOI: https://doi.org/10.1016/i.tca. 2013.01.040 [ Links ]
32. Kupwade-Patil, K.,A1-Aibani, A.F., Abdulsalam, M.F., Mao, C, Bumajdad, A., Palkovic, S.D., Büyükoztürk, O. 2016, Microstructure of cement paste with natural pozzolanic volcanic ash and Portland cement at different stages of curing, Constr BuildMater, 113, 423-441. DOI: https://doi.org/10.1016/i.conbuildmat.2016.03.084 [ Links ]












 uBio
uBio