Revista Boliviana de Química
versión On-line ISSN 0250-5460
Rev. Bol. Quim v.37 n.3 La Paz dic. 2020
DOI: 10.34098/2078-3949.37.3.1
FULL ORIGINAL ARTICLES
Basic Phytochemical screening and antibacterial, antifungal and antioxidant properties
of Syzygium Cumini, a tree from Guyana
Cribado Fitoquímico básico y propiedades antibacterianas, antifúngicas y antioxidantes
de Syzygium Cumini, un árbol de Guyana
Rij Bhushan Tewari *
* Department of Chemistry, Faculty of Natural Sciences, University of Guyana,
Po Box 101110 Georgetown, Guyana. E-mail: brijtew@yahoo.com
Received 03 24 2020 Accepted 08 26 2020 Published 08 26 2020
Abstract
Syzygium cumini or Jamun is an evergreen tropical tree in ornamental plant family Myrtaceae. The plant material (leaves) of Syzygium cumini (Jamun) was collected at the Institute of Applied Science and Technology (IAST), University of Guyana, Turkeyen Campus. Leaves were dried in oven at 50-60 °C for 72 h. The moisture content was calculated. The dried leaves were grounded and extracted in ethanol, methanol, ethyl acétate, and chloroform solvents, succesively. Extracts were collected and evaporation of solvent was done on rotavapour. The respective solvent was added to viscous semi solid liquid extract to make up the desired volume of extract solution. The micro-organisms (Escherichia coli, Staphylococcus aureus and Candida albicans) were obtained from GPHC, Georgetown, Guyana. The antioxidant, antimicrobial and antifungal activity of the different extracts was assessed by methods reported in the literatee. The máximum and the minimum antioxidant power was exhibited by the methanol and the chloroform extracts, respectively. The chloroform and the ethyl acétate extracts were found to have the máximum and the minimum antibacterial activity against Escherichia coli, Staphylococcus aureus, as well as the antifungal activity against Candida albicans, respectively by the disc diffusion method. Phytochemical analysis of the Syzygium cumini leave extracts revealed the presence of carbohydrates, terpenoids, proteins, amino acids and flavo no ids.
Keywords. Syzygium cumini, Antioxidant power, Antifungal activity, Phytochemical screening, antibacterial property.
Resumen
Syzygium cumini o Jamun es un árbol tropical de hoja perenne de la familia de plantas ornamentales Myrtaceae. El material vegetal (hojas) de Syzygium cumini (Jamun) se colectó en el Instituto de Ciencia y Tecnología Aplicadas (IAST) de la Universidad de Guyana, campus de Turkeyen. Las hojas se secaron en estufa a 50-60 °C durante 72 h. Se calculó el contenido de humedad. Las hojas secas se trituraron y se extrajeron en etanol, metano 1, acetato de etilo y disolventes de cloroformo, sucesivamente. Se recogieron los extractos y se evaporó el disolvente en rotavapor. Se añadió el disolvente respectivo al extracto líquido semisólido viscoso para completar el volumen deseado de solución de extracto. Los microorganismos (Escherichia coli, Staphylococcus aureus y Candida albicans) se obtuvieron de GPHC, Georgetown, Guyana. La actividad antioxidante, antimicrobiana y antifúngica de los diferentes extractos se evaluó mediante métodos descritos en la literatura. El poder antioxidante máximo y mínimo fue exhibido por los extractos de metanol y cloroformo, respectivamente. Se encontró que los extractos de cloroformo y acetato de etilo tenían la actividad antibacteriana máxima y mínima contra Escherichia coli, Staphylococcus aureus, así como la actividad antifúngica contra Candida albicans, respectivamente, por el método de difusión por disco. El análisis fitoquímico de los extractos de hojas de Syzygium cumini reveló la presencia de carbohidratos, terpenoides, proteínas, aminoácidos y flavonoides.
Palabras clave. Syzygium cumini, Poder antioxidante, Actividad antifúngica, Screening fitoquímico, Propiedades antibacterianas.
INTRODUCTION
Syzygium cumini or Jamun is an evergreen tropical tree belonging to the flowering plant family myrtaceae. The scientific classification of Syzygium cumini is given as follows:
Syzygium cumini is also known as black plum, java plum, duhat plum and malabar plum. More than one thousand species were classified as belonging to the genus Syzygium. It is a slow growing species. It canreachup to 30 meters and live more than 100 years. Syzygium cumini is original from India, Nepal, Pakistán, Bangladesh, Sri Lanka, Philippines and Indonesia. It was is man-introduced to Florida, USA, Suriname, Brazil, Trinidad &Tobago and Guyana. Syzygium cumini leaves are used as food for live stocks. Wine (and vinegar) are also made from the fruits. It is rich in vitamins A and C. Leaves and bark of S. cumini are used for controlling blood pressure. Syzygium cumini seeds are used in various alternative healing system like Unani, Chínese and Ayurveda medicine. Syzygium cumini wood is water resistance, so it is used in railway sleepers and to installing motors in wells. Kaur et al. [1] investigated drying (50, 70, 90 and - 55° C) characteristics and antioxidant properties of java plum seeds and skin waste. A review on ethnobotanical uses, antimicrobial potential, pharmacological properties and phytoconstituents oí Syzygium cumini was reported by Singh and Navneet [2]. Mitra et al. [3] examined effects of summer, winter, rainy and autumn seasons on UV absorbing properties of the acetone extract of S. cumini leaves pointing out the rainy season as responsible of máximum absorbing properties and it can be regarded as anti-solar agent for preparation of sun screen lotions. The UV absorption properties of the methanolic, ethanolic, benzenic, acetone and ethyl acétate leave extract of S. cumini were examined by Mitra et al. [4]. The acetone leave extract of S. cumini was found to have máximum UV absorption potential. Arunpandian et al. [5] presented a review focused on the information on traditional and medicinal use of S. cumini. Phytochemical contents and antioxidant capacity of S. cumini from Indonesia were investigated by Sukmasari et al. [6]. They have also compared the properties of the leave extract of S. cumini and S. polyanthum. Prema et al. [7] studied the phytochemical constituents and antidiabetic properties of S. cumini seeds extract. S. cumini seeds powder was found to possess antidiabetic properties in type - 2 diabetic model rats. The potential use of S. cumini methanolic leave extract for dengue by increasing platelet and leukocyte levéis were described by Bandida and Corpuz [8]. A review on pharmacological properties and therapeutic potential of S. cumini was discussed by Kumawat et al. [9]. Antimicrobial activity of S. cumini juice extract on enteric pathogenic bacteria (viz. Salmonella typhimurium, Shigella flexneri, Staphylococcus aureus and Escherichia coli) was described by Haque et al. [11]. Khan et al. [12] studied the antifungal potential of the methanolic extract of bark and leaves of S. cumini against Rhizoctonia solani. Kuhn. Hasanuzzaman et al. [13] performed phytochemical screening of methanolic, acetone, chloroform and n-hexane extracts of S. cumini seeds, root, stem, bark, leaves. The antimicrobial potential of methanolic, aqueous, and petroleum ether extracts of S. cumini leaves was tested by Elfadil et al. [14] against bacteria (viz. Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Bacillus subitlis) and fungi {Aspergillus niger and Candida albicans). Ramos and Bandiola [10] has examined phytochemicals in extracts from methanol, ethanol, water, ethyl acétate and hexane macerations out of the leaves of S. cumini. Praphakaran et al. [15] reported that ethanolic extract of leaves and aqueous extracts of seeds S. cumini were found to have very high antimicrobial property for wide range of gram positive and gram negative bacterial strains. The natural antimicrobial potential of extract of all parts of Syzygium cumini (L) over the chemical synthetic antibiotic was studied by Mohit et al. [16]. Jabeen and Javaid [17] evaluated the antifungal potential of the ethanolic, aqueous and n-hexane extracts from leaves, fruit, root bark and stem bark of Syzygium cumini (L) against Ascochyt arabiei, the cause of blight disease of chick pea (Cicer arietinum). The antioxidant activity of S. cumini leaf extracts was investigated using the 2, 2-diphenyl-l-picrylhydrazyl (DPPH) free radical-scavenging and ferric-reducing antioxidant power (FRAP) arrays was described by Rúan et al. [18]. A significant linear relationship between antioxidant potency, free radical-scavenging ability and the content of phenolic compounds of leaves extracts were observed. The plant of Syzygium cumini with fruits is shown in Figure 1.
RESULTS AND DISCUSSION
Moisture contents
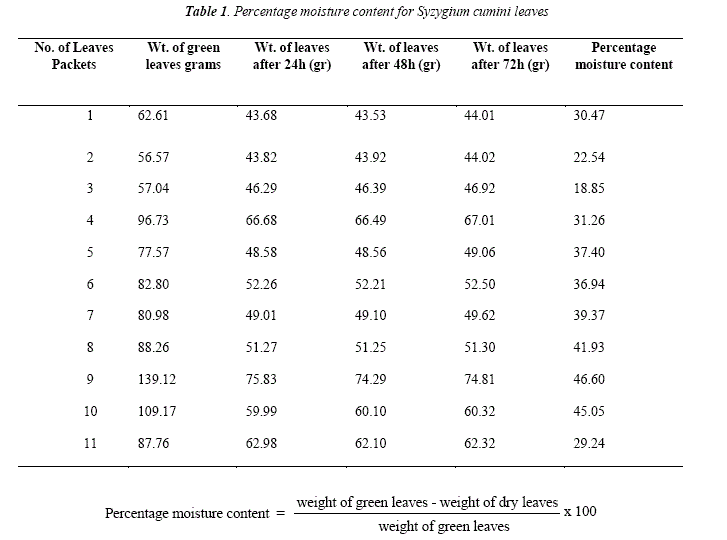
The moisture contents were calculated and are exposed in Table 1
Reducing power
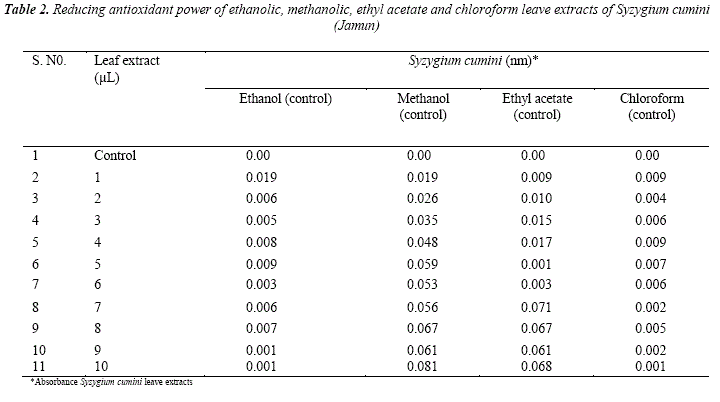
The reducing power serves to measure the reductive ability of an antioxidant, and it is evaluated by the transformation of Fe(III) to Fe(II) in the presence of the sample extracts, Gulcin et al. [19]. The reducing power oíSyzygium cumini leaves extracts are summarized in Table 2. From Table 2 it is clear that reducing power increased with an increase in the concentration of plant extracts. The ability to reduce Fe (III) may be attributed to hydrogen donation from phenolic compounds, according to Shimada et al. [20], which is also related to the presence of the reducing agent, Duh [21]. In addition, the number and position of the hydroxyl group of phenolic compounds also rule their antioxidant activity, Sawaddiwong et al. [22]. Some deviation from the increase in the reducing power of leave extract with an increase in concentration may be due to decrease in hydrogen do ñor ability of phenolic compounds.
It is observed from Table 2 that the antioxidant power of Syzygium cumini leaves extract in various solvents follow the order:
Methanol > ethyl acetate >ethanol > chloroform
In the ethanol extract high absorbance was observed at 1.0 μL concentration. From 2.0 to 10.0 μL concentration no defínite order of reducing antioxidant power was observed. The methanol extract showed high reducing anti-oxidant power or high difference in the absorbance of leaves extract and control. In ethyl acétate extract máximum difference in the absorbance of leaves extract and control was observed at 7.0 μL concentration. In chloroform extract minimum reducing or antioxidant power or minimum difference in absorbance of leaves extract and control was observed.
Antitnicrobial activity
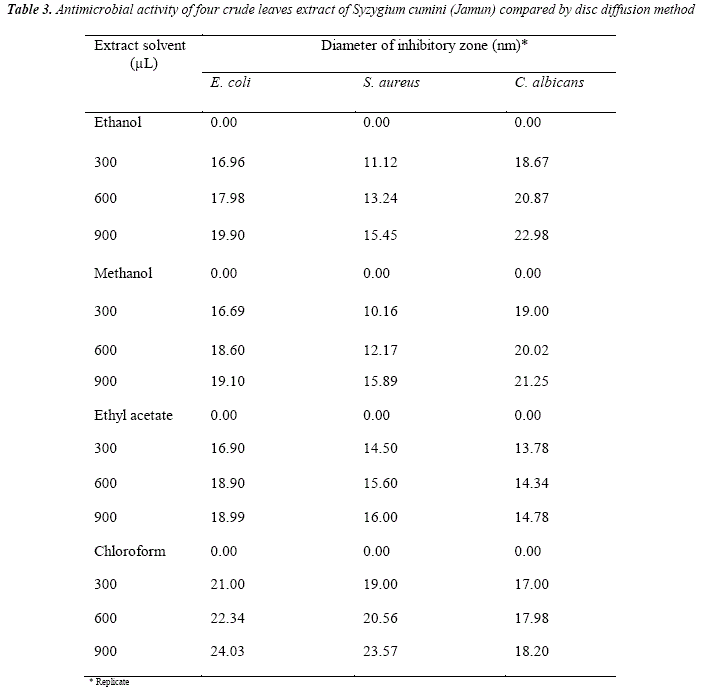
Antimicrobial activity of Syzygium cumini leaves extract against gram positive and negative bacteria and Candida albicans fiingus are summarized in Table 3 and in the Table 4 by disc diffusion and poison píate methods, respectively. The results in Table 3 in disc diHusion and Table 4 poison plate methods indicated that all plants extracts showed that antimicrobial activities toward the gram positive bacteria Staphylococcus aureus as well as gram negative bacteria Escherichia coli and Candida albicans.
It is observed from Table 3 that inhibitory zone follows the order:
Chloroform > ethanol > methanol > ethyl acetate
Chloroform extract showed more effective inhibitory zone against bacteria and fiingi in comparison to other solvents extracts. In ethanol extract máximum (22.98 nm; 900 μL) and minimum (11.12 nm; 300 μL) inhibitory zone was observed for Candida albicans and Staphylococcus aureus, respectively. In methanol extract máximum (21.25 nm; 900 μL) and minimum (10.16 nm; 300 μL) inhibitory zone observed for Candida albicans and Staphylococcus aureus, respectively. In ethyl acétate extract máximum (18.99 nm, 900 μL) and minimum (13.78 nm; 300 μL) inhibitory zone was observed for Escherichia coli and Candida albicans, respectively. In chloroform extract máximum (24.03 nm, 900 μL) and minimum (17.00 nm, .00 μL) inhibitory zone observed for Escherichia coli and Candida albicans, respectively.
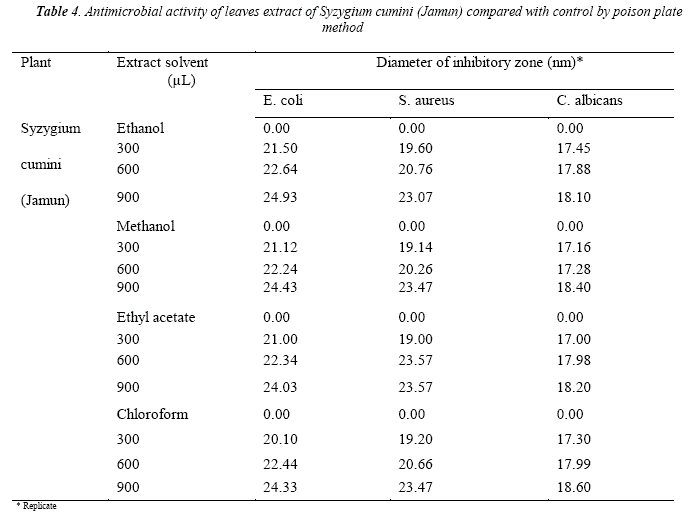
It is clear from Table 4 that inhibitory zone follows the order:
Ethanol > ethyl acetate > chloroform > methanol
All extracts showed nearly same inhibitory zone or antibacterial activity. In ethanol extract máximum (24.93 nm; 900 μL) and minimum (17.45 nm; 300 μL) inhibitory zone was observed for Escherichia coli and Candida albicans, respectively. In methanol extract máximum (24.43 nm; 900 μL) and minimum (17.16 nm; 300μL) inhibitory zone was observed for Escherichia coli and Candida albicans, respectively.
In ethyl acétate extract máximum (24.03 nm; 900 μL) and mínimum (17.00 nm; 300 μL) inhibitory zone was observed for Escherichia coli and Candida albicans, respectively. In chloroform extract máximum (24.33 nm; 900 μL) and mínimum (17.30 nm; 300 μL) inhibitory zone was observed for Escherichia coli and Candida albicans, respectively.
Phytochemical analysis
Phytochemical analysis Table 5 of the Syzygium cumini (Jamun) leaves extracts revealed the presence of carbohydrates, flavonoids, terpenoids, saponins, tannins, proteins and amino acids etc.
It is observed from Table 5 that Phyto-constituent (carbohydrate) is present in the extract of each solvent. Alkaloids are not detected in all four (ethanol, methanol, ethyl acétate and chloroform) solvent extracts. Glycoside is not detected in the ethanol solvent extract. Glycosides tests for methanol, ethyl acétate and chloroform extracts could not be performed. Saponins are only tested in methanol solvent. Tannins and flavonoids are tested in ethanol extracts only. Ethanol solvent is found to be more efíective solvent for the phytochemical screening of Syzygium cumini leaves extract.
CONCLUDING REMARKS
(i) Syzygium cumini leave extracts were found to have antimicrobial, antifiingal and antioxidant properties.
(ii) The antioxidant power oí Syzygium cumini 's leaves in extracts in various solvents follows the order:
methanol> ethyl acetate > ethanol> chloroform
(iii) The antioxidant power of Syzygium cumini 's leaves in extracts in various solvents was found to increase with increase of its concentrations.
(iv) The chloroform extract was found to have more antibacterial property in comparison to methanol, ethylacetate, and ethanol extracts.
(v) In disc diffiision method inhibitory zone for antibacterial activity follows the order:
Chloroform > ethanol > methanol > ethyl acetate
(vi) In poison píate method inhibitory zone for antibacterial activity follows the order:
Ethanol > ethyl acetate > chloroform > methanol
(vii) In poison píate method all extracts have showed nearly same inhibitory zone or antibacterial activity.
EXPERIMENTAL
Collecting of plant material
The plant material (leaves) of Syzygium cumini was collected at the Institute of Applied Science and Technology (IAST), University of Guyana, Turkeyen Campus, Georgetown. Guyana.
Preparation ofplant materials:
The collected leaves sample oí Syzygium cumini was weighted on Citizen CTG 3000E electronic balance. The leaves dried in oven (Gallenhamp Incubator Model IH-150) at 50-60°C. The dried leaves were cooled at room temperature and weighted again on Citizen electronic balance. Weight of green leaves, dried leaves and valué of percentage moisture content invarious samples oí Syzygium cumini is givenin Table 1. The weight of ground leaves oí Syzygium cumini was found to be 560 grams.
Collection oftest organism
Three micro-organisms (Escherichia coli, Staphylococcus aureus and Candidus albicans) were used for the study. All the tested strains are reference strains and were collected from the microbiology laboratory of Georgetown Public Hospital Corporation, Georgetown (GHPC). All cultures are maintained in nutrient broth (Himedia, M002) at 37°C and maintained on nutrient agar (Himedia MM012) slants at 4°C.
Extraction and preparation of test solutions
The grounded leaves of Syzygium cumini were extracted in each solvent, ethanol, ethyl acétate, chloroform and methanol. 20 g of dried pulverized leaves were soaked with 200 mL of each solvent for 48 h separately. Solvent is decanted each time and residue again soaked with same solvent for 24 h. The total extract is combined and filtered. The evaporation of solvent was done on rotavapour (Buchi). The respective solvent was added to viscous semi solid liquid extract to make up the desired volume of extract solution.
Reducing antioxidant power
Ammonium thiocyanite, ferric chloride, linoleic acid (99.5 %), thiobarbituric acid, trichloroacetic acid, potassium ferrocyanide, butylated hydroxyl anisol, sodium dihydrophosphate, sodium monhydrophosphate, potassium dihydrophosphate obtained from Aldrich. The reducing antioxidant power of the plants methanolic, ethanolic, ethyl acétate and chloroform extract was determined by the method reported in the literature [23,24]. Different concentration of plant extracts (100 - 1000 μL) in 1 mL of distilled water were mixed with phosphate buffer (2.5 mL, 0.2 M ,pH 6.6) and potassium ferricynaide K3[ Fe(CN)6] , (2.5 mL,1 %) . The mixture was incubated at 50° C for 20 min. Then 2.5 mL of trichloroacetic acid 10 % was added to mixture, which was then centrifuged for 10 min at 3000 rpm. The upper layer (2.5 mL) was mixed with distilled water 92.5 mL and FeCb, 0.5 mL, 1 %. The absorbance was measured at 700 nm against a blank using UV-Vis spectrophotometer (Phillips X500). Increased absorbance of the reaction mixture indicates increase in the reducing power.
Anti-Microbial Assay
Materials
Mueller Hinton Agar, Agar plates and microbial discs were purchased from the International Pharniaceutical Association (IPA) in Guyana. Solvents like chloroform, ethanol, ethyl acétate and methanol obtained from Aldrich. Scitilation vials 20 mL were obtained from Meditron Scientific Sales, Georgetown, Guyana.
Aseptic chamber
Aseptic chamber consists of a wooden box of L = 1 meter, B = 1 meter and D = 0.5 meter área. Chamber is cleaned with 70 % ethanol twice and irradiated with short wave UV light for 1 h.
Potato dextrose agar (PDA) médium
Potato Dextrose Agar (PDA) médium prepared according to method reported by Talara [25]. This is the médium on which cultured bacteria Escherichia coli and Staphylococcus aureus were grown. The 200 g potato was peeled finely chopped and boiled to a mash in distilled water. Each 12.5 g dextrose and 12.5 g agar was placed in a 1 L measuring cylinder. The potato mash was stirred and poured in to the same measuring cylinder. Distilled water was added to make the solution up to 500 mL. The content was stirred until the consistency of solution mixture. The stirred mixture poured into conical flasks, plugged with cotton wool and tightly wrapped by aluminum foil. The flasks were autoclaved at 121° C, 15 psi, for 15 minutes.
Mother plates
Mother plates were prepared by pouring PDA mixture into Petri dishes and to cool at room temperature, in the aseptic chamber.
Antimicrobial assay was done by disc diffusion and poisons píate method [26] using plants Syzygium cumini extracts (ethanol, methanol, ethyl acétate and chloroform) and commonly used antibiotics. The test quantities of specific extracts were dissolved in depending upon the solubility of the extracts. The dissolution of the organic extracts (chloroform, methanol, ethanol and ethyl acétate) was aided by water, which did not affect the growth of microorganism, in accordance with our control experiments. The surfaces of media were inoculated with bacterial from a broth culture. After 18 h of incubation at a specific temperature 28° C - 30° C for Escherichia coli, Staphylococcus aureus and Candidus albicans the plates were examined and the diameters of the inhibition zones were measured to the nearest millimeter.
Phytochemical analysis of the plant extracts
Materials
Glacial acetic acid, thyonil chloride, dichloromethane, copper sulfate, lead acétate, diethyl ether, ferric chloride, acetic anhydride, antimony chloride, all obtained from Aldrich.
Method
Phytochemical analysis of all the aqueous plant extracts was carried out by suitable methodologies in search of active ingredient responsible for antimicrobial toxicity. The phytochemicals include under study were saponins, terpenoids, alkaloid, glycoside, carbohydrates, protein and amino acids, tannins, and flavonoids the analysis was carried out according to the methodologies of Edeoga et al. [27].
REFERENCES
1. Kaur, A., Singh, D. Kaur, H. , Sogi, J. 2018, StoredProducts Postharvest Res., 9 (4), 36. [ Links ]
2. Navneet, S.A., 2018, IJIPSR, 6 (1), 32. [ Links ]
3. Mitra, P., Mitra P.K , Ghosh, T. 2018, Acta Sci. Agri., 2 (11), 59. [ Links ]
4. Mitra, P., Mitra P.K., Ghosh, T. 2018, Acta Sci. Param Sci., 2 (9), 69. [ Links ]
5. Aranpaudiyan, J., Roselin, R.B., Chitra, S.R., Kaniga, P. 2018, WIJJPS, 7(4), 499. [ Links ]
6. Sukmasari, S., Mohd, F.N., Doolaanea, A.A., Jabbar, O.A., Qader, A., Rahman, M.N.A., 2018, J. Pharm. Sci. & Res. 10 (1), 31. [ Links ]
7. Proma, N.M., Naima, J., Islam, J.A., Papel, M.R., Rahman, M., Hossain, M.K. 2018, IJIPSR, 9 (5), 1806. [ Links ]
8. Bandida, T.M.B., Carpuz, M.J.T. 2018, Pharm. Anal. Acta., 9 (5), 1. [ Links ]
9. Kumawat, M., Damar, J., Kachchhwaha, J., Garg A. K., Singh C. , 2018, WJPPS, 7(03), 312. [ Links ]
10. Ramos, I.L., Bandida, T.M.B. 2017, DerPharm. Lett, 9 (2), 74. [ Links ]
11. Haque, R., Sumiya, M.K., Sakib, N., Sarkar, O.S., Siddique, T.T.T., Hossain, S., Islam, A., Parvez, A.K., Talukder, A.A, Dey, S. K. 2017, Adv. Microbiol. 7, 193. [ Links ]
12. Khan, A., Jabeen, K., Iqbal, S., 2016, PureAppl. Biol, 5 (2), 193. [ Links ]
13. Hasanuzzaman, M., Islam, U., Islam, M.B. 2016, J. Biol. Sci., 24, 11. [ Links ]
14. Elfadil, A.G., Karamallah, A.A., Abualhassan, A.M., Hamid, A.A., Sabahelkhier, M.K. 2015, NovaJ. Med. Biol. Sci., 4 (2), 1. [ Links ]
15. Prabhakaran, S., Gothandam, K.M., Sivashanmugam, K. 2011, Res. Pharm., 1 (11), 22. [ Links ]
16. Mohit, Y., Kadtan, K., Wakte, P. 2011, J. Pharm. Res., 4(8), 278. [ Links ]
17. Jabeen K., Javaid, A. 2010, Nat. Prod. Res., 24 (12), 1158. [ Links ]
18. Rúan, Z.P., Zhang, L.L., Lin, Y.M. 2008, Maléenles, 13, 2545. [ Links ]
19. Gulcin, L., Oktay, M., Kirecci, E., Kufrevioglu, O.I. 2003, Food Chem., 83 (3) ,371. [ Links ]
20. Shimada, K., Fujikawa, K., Yahara, K., Nakamura, T. 1992, J. Agri. Food Chem., 40, 945. [ Links ]
21. Duh, P. 1998, J. Am. OH Chem. Soc. 75 , 455. [ Links ]
22. Sawaddiwong, R., Jongjareorak, A., Bazakul, S. 2008, KMITL Sci. J., 8 (2), 54. [ Links ]
23. Oyaizu, M. 1986, Jpn. J. Nutr., 44, 307. [ Links ]
24. Huda-faujan, H. Noriham, A., Narrakaih, A.S., Badji, A. 2009, SAfr. J. Biotechnol, 8 (3), 484. [ Links ]
25. Talaro, K.P., Wm. C. Brown Publishers, USA, 1993. [ Links ]
26. Awoyinka, A.O., Balagun, A.O., Ogunnowo, A. A. 2007, J. Med. PlantsRes., 7,063. [ Links ]
27. Edeoga , H.O., Okwu, D.E., Mbalble, B.O. 2005, Afr. J. Biotechnol, 4 (7), 685. [ Links ]











 uBio
uBio