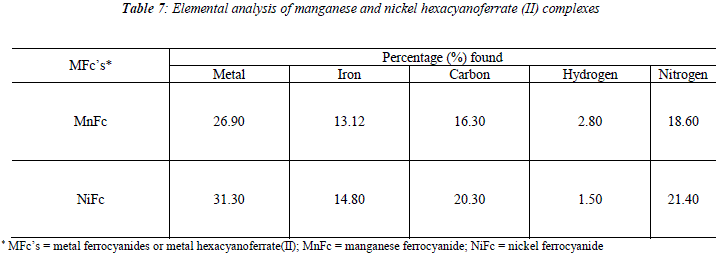Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Boliviana de Química
On-line version ISSN 0250-5460
Rev. Bol. Quim vol.33 no.5 La Paz Dec. 2016
ARTÍCULOS ORIGINALES
Interacción of 2-aminophenol, 4-aminophenol, 2-nitrophenol and 4-nitrophenol with manganese and nickel hexacyanoferrate (II) complexes
Savi Bhalkaran1,2, Brij B. Tewari1,*
*Corresponding author: brijtew@yahoo.com
Abstract
The adsorptive interaction of 2-aminophenol (2 - AP), 2 - nitrophenol (2 - NP), 4 - aminophenol (4 - AP) and 4 -nitrophenol (4 - NP) with manganese and nickel ferrocyanides have been studied at neutral (pH 7.01 ± 0.01) and at thermal (T° 30 ±1oC) conditions. The progress of adsorption was followed spectrophotometrically by measuring the absorbance of amino acids solution at their corresponding ![]() . The Langmuir type of adsorption is followed in the concentration range of 10-4 to 10-5 mol/L of substituted phenol solutions. Nickel ferrocyanide was found to have greater adsorption ability in comparison to manganese ferrocy anide with all four substituted phenol adsorbates. 4 -Nitrophenol and 2 - aminophenol was found to have greater and lesser affinity, respectively with both metal ferrocyanides studied.
. The Langmuir type of adsorption is followed in the concentration range of 10-4 to 10-5 mol/L of substituted phenol solutions. Nickel ferrocyanide was found to have greater adsorption ability in comparison to manganese ferrocy anide with all four substituted phenol adsorbates. 4 -Nitrophenol and 2 - aminophenol was found to have greater and lesser affinity, respectively with both metal ferrocyanides studied.
Keywords: Removal, 2-Aminophenol, 2-Nitrophenol, 4-Aminophenol, 4-Nitrophenol, Adsorption, Metal ferrocyanides, Methylene blue dye.
Resumen
Spanish title: Interacción de 2-aminofenol (2AF), 2-nitrofenol (2NF), 4-aminofenol (4AF) y 4-nitrofenol (4NF) con complejos de hexacianoferrocianuro (II) de manganeso y níquel. La interacción adsorbativa del 2-aminofenol (2AF), 2-nitrofenol (2NF), 4-aminofenol (4AF) y 4-nitrofenol (4NF) con ferrocianuro de manganeso y níquel fue estudiada en condiciones de neutralidad (pH 7.01 ± 0.01) y en condiciones térmicas (T° 30 ± Io C). El progreso de adsorción fue monitoreado espectrofotométricamente por la medida de la absorbancia de la solución de aminoácidos y su correspondiente ![]() . La adsorción del tipo de Langmuir es controlada por la concentración de las soluciones de los fenoles sustituidos en el rango 10-4 to 10-5. mol/L. El ferrocianuro de níquel mostró una mayor habilidad adsorbativa en comparación con el ferrocianuro de manganeso con los cuatro adsorbatos fenólicos. El 4-Nitrofenol y el 2-aminofenol mostraron tener mayor y menor afinidad respectivamente con ambos ferrocianuros metálicos estudiados.
. La adsorción del tipo de Langmuir es controlada por la concentración de las soluciones de los fenoles sustituidos en el rango 10-4 to 10-5. mol/L. El ferrocianuro de níquel mostró una mayor habilidad adsorbativa en comparación con el ferrocianuro de manganeso con los cuatro adsorbatos fenólicos. El 4-Nitrofenol y el 2-aminofenol mostraron tener mayor y menor afinidad respectivamente con ambos ferrocianuros metálicos estudiados.
INTRODUCTION
Phenolic compounds, also referred to as 'phenols', present in water destroy the environment because of their toxicity. Phenols are a family of organic compounds that are characterized by a hydroxyl (-OH) group attached to a sp2 hybridized carbón atom that is part of an aromatic ring [1]. Phenol and its derivatives are used in a wide range of industrial processes such as the manufacture of pesticides, dyes, explosives and drugs and they also have agricultural utility as fungicides, insecticides and herbicides. The manufacture of these substances is largely responsible for the increasing presence of phenolic compounds in water and groundwater [2].
When the concentrations of phenols are as low as parts per billion (ppb) levéis, they can adversely affect the taste and odor of drinking water [3]. Phenolic compounds not only adversely affect plants and animáis directly but also indirectly in that they may be linked to soil sickness. These phytotoxic substances may cause delayed and poor growth of plants and delayed bearing of fruits when there is long-term cultivation of plants of the same species in that área. This would imply that high levéis of phenolic compounds are not favorable to plant growth [4].
The removal of phenolic compounds from water has received much study because of the danger posed by these compounds to human health and ecosystems in water reservoirs. Some of the strategies used to remove phenols include the use of sorbents such as white radish (Raphanus sativus) peroxidase in the presence of polyethylene glycol [5], ferrate (VI) [6], commercial activated carbón [7], macroreticular polymers based on cross-linked styrene and acrylate systems [8] and rice husk ash [9]. Other systems used include catalytic removal of phenolic contaminants by ozone using manganese and cerium oxides [10], and using a semifluidized bed bio-reactor [11]. The use of adsorption in removing organic contaminants from wastewater is a good and widely used technique because it is a recuperative rather than a destructive technique [12] and as such it is a distinct possibility that the adsorbed phenols may be recovered. The use of metal cyano complexes for the removal of organic molecules has been extensively studied [13-20].
For the ferrocyanides of divalent metal ion, a general formula M2[Fe(CN)6].nH2O has been reported, where M2+ represents to an exchangeable divalent transition metal ion [21]. Metal ferrocyanides are the examples of tightly bounded low spin complexes. In octahedral field, the five-fold degenerate levéis split into a three-fold level (t2g) and a doubly degenerate levéis (eg) of higher energy. The splitting valué (10 Dq) for metal ferrocyanides is 30 - 3200 cnr1. Thus, electronic configuration of Fe is t62g as all the 3d6 electrons become paired due to strong field behavior of CN- ligand. Besides ![]() donation from CN- to Fe, there is back donation of
donation from CN- to Fe, there is back donation of ![]() electrons from its
electrons from its ![]() orbital to anti-bonding
orbital to anti-bonding ![]() orbital of CN- ligand. The back bonding accounts for high splitting valúes and reduces the shielding of 3s orbitals, which indicates the high electrón density on the nucleus. The structure and spectra of metal ferro and ferrocyanides has been studied by a number of workers [22]. The transition metal ferrocyanides generally exists in a polymeric lattice structure with [Fe (CN)]4- anions and other transition metal ions present in the lattice is coordinated through the nitrogen end of the cyanide ligand.
orbital of CN- ligand. The back bonding accounts for high splitting valúes and reduces the shielding of 3s orbitals, which indicates the high electrón density on the nucleus. The structure and spectra of metal ferro and ferrocyanides has been studied by a number of workers [22]. The transition metal ferrocyanides generally exists in a polymeric lattice structure with [Fe (CN)]4- anions and other transition metal ions present in the lattice is coordinated through the nitrogen end of the cyanide ligand.
A search of literatee indicated that several studies have been done for adsorption and removal of substituted phenols [23-32] but few work has been done on the removal of phenols using metal cyano complexes as adsorbent. In view of this attempt has been made to investígate ferrocyanide - phenol system. In addition, present work describes a removal of 2 - aminophenol, 4 - aminophenol, 2 - nitrophenol and 4 - nitrophenol through adsorptive interaction with nickel and manganese ferrocyanides. The aminophenols and nitrophenols were chosen as representative phenols due to electrón - donating and withdrawing groups, respectively.
RESULTS AND DISCUSSION
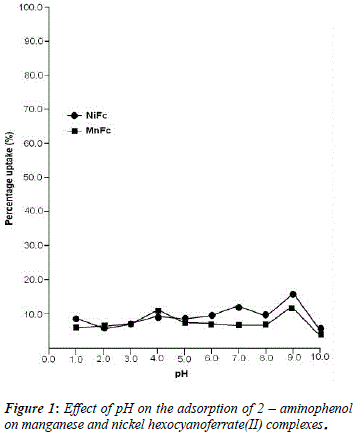
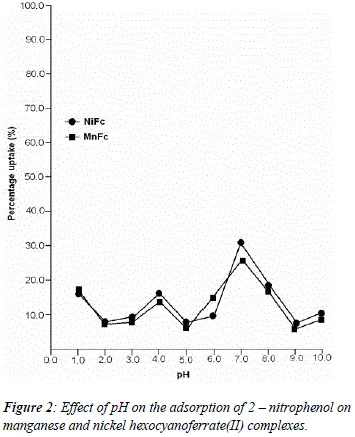
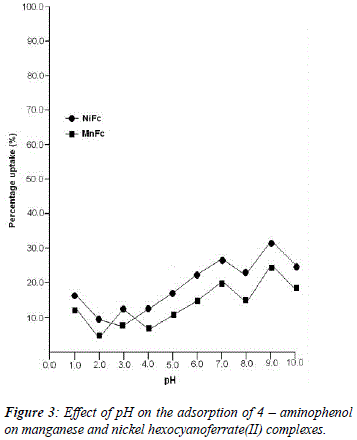
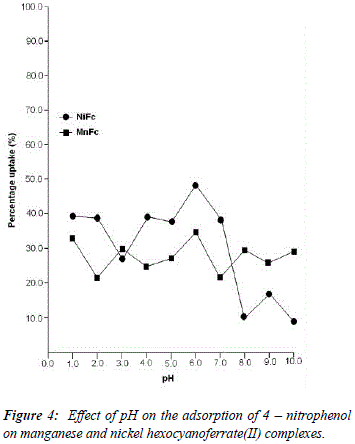
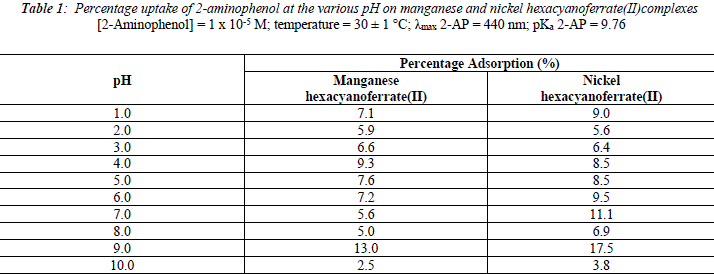
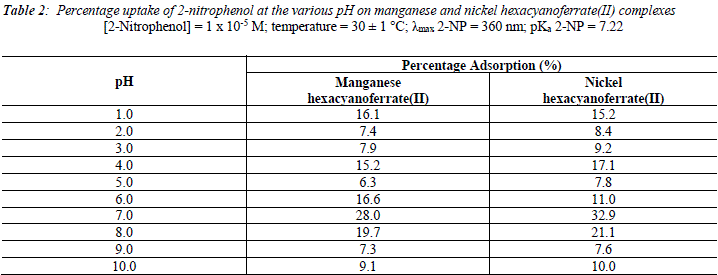
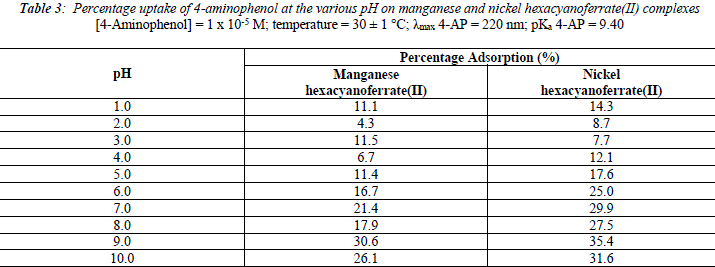
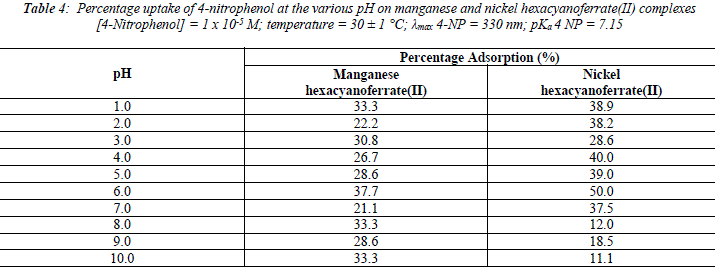
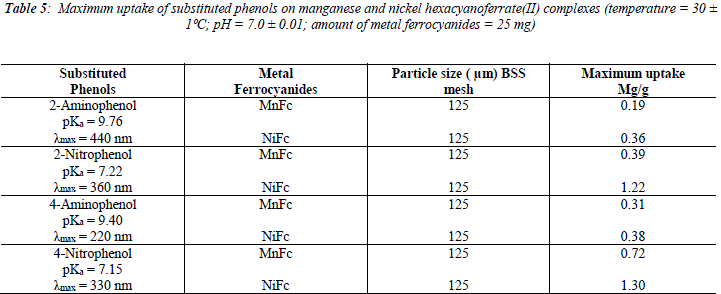
The uptake of 2 - AP, 2 - NP, 4 -AP and 4 - NP as function of pH on metal ferrocyanides was studied over a pH range of 2.0 - 10.0 (Figures 1, 2, 3, 4). The percentage uptake of 2 - AP, 2 - NP, 4 -AP and 4 - NP on manganese and nickel ferrocyanides are given in Table 1, 2, 3 and 4 respectively. Percentage binging of substituted phenols has been calculated with the help of optical density of substituted phenols solution before and after adsorption, corresponding to saturation point.

Adsorption of 2 - aminophenol (pKa = 9.76), 2 - nitrophenol (pKa = 7.22), 4 - aminophenol (pKa = 9.40) and 4 -nitrophenol (pKa = 7.15) are found máximum near to their respective pKa valúes. In general, the Figures 1 - 4 showed high percentage adsorption at pH valúes 6.0, 7.0 and 9.0 and 9.0 for 4-NP, 2-NP, 2-AP and 4-AP, respectively. Above the pKa valué of the phenols, the phenolate ion would be predominant. With the negative charge on phenolate ion seems to be repelled by the surface charge of ferrocyanides, causing less adsorption than in the case of dissociated phenols at pH valúes lower than pKa [33]. Since the 2-AP and 4-AP had pKa valúes above neutral pH (7.0) the high adsorption occurred around more basic pH range, while 2-NP and 4-NP have pKa valúes around neutral pH (7.0) so high adsorption occur at neutral pH range.
It is observed from Tables 1, 2, 3 and 4 that highest adsorption of substituted phenols on metal ferrocyanides follow the order:
![]()
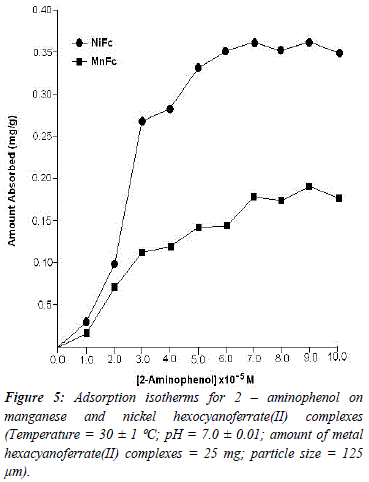
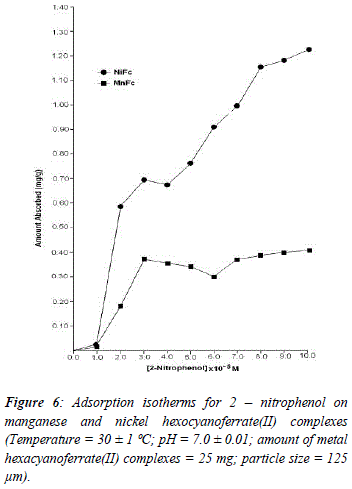
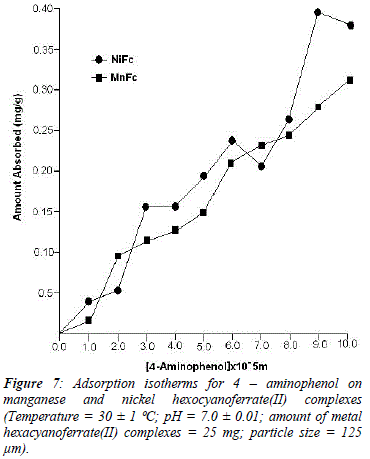
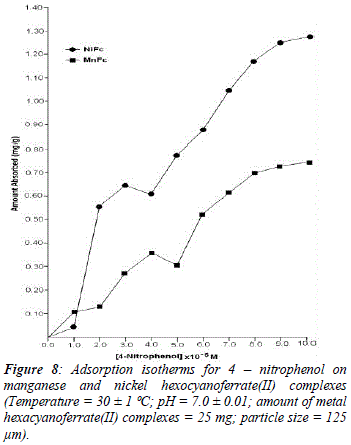
The reason for difference in adsorption of substituted phenols on metal ferrocyanides may be due to substituents attached to the aromatic ring. Since the presence of the OH group is common in all substituted phenols, higher adsorption in the case of nitrophenols is only due to nature of substituents. Electron density of aromatic ring is strongly influenced by the nature of substituents attached to it. The nitro group is electrón withdrawing and reduces the overall electrón density in the % system of benzene ring. On the other hand, the amino group is electrón -donating, which enhances the electrón density of benzene ring. The lesser adsorption in case of ortho-substituted phenols in comparison to para-substituted phenols may be due to the presence of intramolecular hydrogen bonding. Due to presence of intramolecular hydrogen bonding in ortho-substituted phenols lesser number of sites available on it for bonding with metal ferrocyanides, in comparison to para-substituted phenols. Figures 5, 6, 7 and 8 shows the adsorption isotherms of 2-AP, 2-NP, 4-AP and 4-NP studied on manganese and nickel ferrocyanides. All isotherms are positive and concave to the concentration axis. The graph shows that adsorption is rapid at lower concentrations, then shows down at higher concentration and level off. This suggests that there is saturation of the adsorbent, where all possible adsorption sites on the metal ferrocyanides are filled. The valúes of máximum uptake of substituted phenols on metal ferrocyanides are given in Table 5. The following order of máximum uptake of 2-AP, 2-NP, 4-AP and 4-NP on metal ferrocyanides was observed:
![]()
The present trend is justified by assuming that adsorption is directly proportional to the Specific Surface Área (SSA) of metal ferrocyanides. The SSA of metal ferrocyanides was calculated by basic methylene blue dye adsorption. The SSA of manganese and nickel ferrocyanides for 125 ![]() BSS mesh size were found to be 185.54 and 216.43 m2/g, respectively.
BSS mesh size were found to be 185.54 and 216.43 m2/g, respectively.
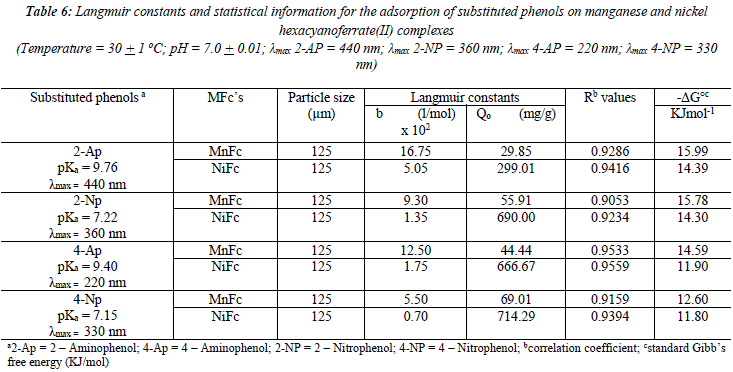
Langmuir plots (l/qeq versus 1/Ceq) for adsorption of 2-AP, 2-NP, 4-AP and 4-NP on manganese and nickel ferrocyanides was constructed (Figures not provided). The data was analyzed in terms of the linear form of Langmuir equation [34].
![]()
Where Ceq is equilibrium concentration of phenol, b is a constant related to the equilibrium constant or bonding energy (b ![]()
![]() the parameter b reflects the steepness of the approach to saturation; more precisely, the b valué is the reciprocal of concentration at which half of the saturation of the adsorbent is attained), Qeq is the amount (mg) of adsórbate adsorbed per gram of adsorbent and Q° is the adsorption máxima, i.e., mg of phenol required per gram of metal ferrocyanide for forming a complete monolayer on the surface. Linear nature of Langmuir plots confirms the formation of mono layer of 2-AP, 2-NP, 4-AP and 4-NP on the surface of metal ferrocyanides. The valúes of Langmuir constants b and Q° for all the systems were obtained from the slope and intercept of the plots, respectively. Their calculated valúes are given in Table 6. The Q° valué is found to be a máximum for NiFc - 4 NP system, while it is a minimum for the MnFc - 2AP system, while the b valúes are found to be a máximum for the MnFc - 2AP system and a minimum of the NiFc - 4 AP system.
the parameter b reflects the steepness of the approach to saturation; more precisely, the b valué is the reciprocal of concentration at which half of the saturation of the adsorbent is attained), Qeq is the amount (mg) of adsórbate adsorbed per gram of adsorbent and Q° is the adsorption máxima, i.e., mg of phenol required per gram of metal ferrocyanide for forming a complete monolayer on the surface. Linear nature of Langmuir plots confirms the formation of mono layer of 2-AP, 2-NP, 4-AP and 4-NP on the surface of metal ferrocyanides. The valúes of Langmuir constants b and Q° for all the systems were obtained from the slope and intercept of the plots, respectively. Their calculated valúes are given in Table 6. The Q° valué is found to be a máximum for NiFc - 4 NP system, while it is a minimum for the MnFc - 2AP system, while the b valúes are found to be a máximum for the MnFc - 2AP system and a minimum of the NiFc - 4 AP system.
The standard molar or Gibbs free energy ![]() for adsorption of substitution phenols on metal ferrocyanides has been calculated using the equation:
for adsorption of substitution phenols on metal ferrocyanides has been calculated using the equation:
![]()
Where K is the equilibrium constant at temperature T and R is gas constant. The calculated valúes of ![]() are given in Table 6. The negative Gibbs free energy valúes indícate feasibility of the process and spontaneous nature of the adsorption. The correlation coefficient (R) is calculated by regression analysis and valúes are given in Table 6. The overall statistics are excellent with an average correlation coefficient valué of 0.9382. Metal ferrocyanides bonded with phenolic pollutants are disposed of an incinerator.
are given in Table 6. The negative Gibbs free energy valúes indícate feasibility of the process and spontaneous nature of the adsorption. The correlation coefficient (R) is calculated by regression analysis and valúes are given in Table 6. The overall statistics are excellent with an average correlation coefficient valué of 0.9382. Metal ferrocyanides bonded with phenolic pollutants are disposed of an incinerator.
CONCLUDING REMARKS
The following conclusions can be drawn from the present study.
1. Results in the present research work explore the possibility of use of metal hexacyanoferrate(II) complexes for the removal of substituted phenols from the environment.
2. Results also show that adsorption of substituted phenols on metal ferrocyanides is quite satisfactory.
3. Nickel ferrocyanides were found to have greater adsorption of all adsorbates than manganese ferrocyanides.
4. 4-NP and 2-AP were found to have greater and lesser affinity respectively with both adsorbents.
5. The most effective pH for the removal of 2-AP, 2-NP, 4-AP and 4-AP was found to be 9.0, 7.0 9.0 and 6.0, respectively.
6. The high correlation coefficient valúes indícate high affinity between the metal ferrocyanides surface and substituted phenols.
EXPERIMENTAL
Chemicals
Potassium ferrocyanide, manganese(II) chloride, nickel(II) chloride, 2 - aminophenol, 4 - aminophenol, 2 -nitrophenol and 4 - nitrophenol were obtained from BDH, Poole, England. All chemicals used were of Analytical Reagent Grade and used without further purification. All solutions were prepared in double distilled water.
Synthesis of metal ferrocyanides
Nickel and manganese ferrocyanides were prepared by adding metal chlorides (500 mi, 0.1 M) and potassium ferrocyanide (167 mi, 0.1 M) solutions with constant stirring [35]. The reaction mixture was heated on a water bath for 3 h and kept as such at room temperature for 24h. the precipitate was filtered under vacuum, washed several times with distilled water and dried in an air oven at 60 °C. The dried product was grounded and sieved to 125 (im particle size.
Characterization of metal ferrocyanides
Manganese and nickel ferrocyanides are amorphous solid which gave no x-ray line. Both metal ferrocyanides are characterized by elemental analysis and spectral studies. The percentage composition of manganese, nickel and iron were determined with the help of IL - 751 atomic absorption spectrophotometer. CHN analysis was carried out with a CEST-118, CHN analyzer. The valúes are given in Table 7.
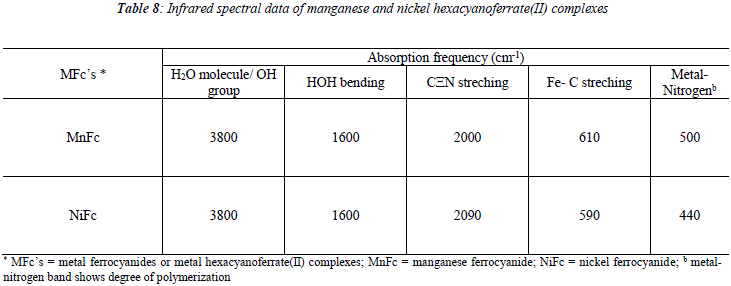
Infrared spectra of the metal ferrocyanides were recorded in KBr disc on Beckman IR-20 spectrophotometer. Both metal ferrocyanides show a broad peak at 3800 cm-1 characteristics of water molecule and OH group. Another peak at 1600 cm-1 is due to HOH bending. Two sharp bands at around 2000 cm-1 and 600 cm-1 are characteristic of cyanide and Fe-C stretching, respectively. Another sharp band at around 500 cm-1 probable shows the presence of metal nitrogen band due to polymerization. The infrared spectral data are given in Table 8.
Adsorption studies
Effects ofpHon substituted phenols on metal ferrocyanides
The adsorption of substituted phenols on metal ferrocyanides as a function of pH range (1.0 - 10.0) and concentration (10-5 M) of adsorbates was studied at room temperature 30 ± 1 °C. Appropriate buffers were used to achieve desired pH.
The buffers used were 0.2 M potassium chloride and 0.2 M hydrochloric acid for pH 1.0 and 2.0; 0.1 M potassium hydrogen phthalate and 0.1 M hydrochloric acid for pH 3.0 to 4.0; 0.1 M potassium hydrogen phthalate and 0.1 M sodium hydroxide for pH 5.0; 0.1 M potassium dihydrogen phosphate and 0.1 M sodium hydroxide for pH 6.0 and 7.0; 0.025 M bórax and 0.1 M hydrochloric acid for pH 8.0; 0.025 M bórax and 0.1 M sodium hydroxide for pH 9.0 and 10.0. The pH of each solution was verified using a pH meter (Lamotte Chemical Company). It was found that species of buffers used do not absorb on metal ferrocyanides. This was checked by conductivity measurements. A series of 15 mL test tubes were employed for the adsorption studies. 10 mi of buffered phenol was placed into test tubes and 25 mg of metal ferrocyanide was added. The test tubes were then stoppered and agitated for 6 hours until equilibrium was obtained. The contents were then centrifuged at 3000 rpm for 15 minutes, decanted and the absorbance of 2-NP, 4-NP, 2-AP and 4-AP solutions after adsorption was measured spectrophotometrically at their corresponding ![]() 360 nm, 330 nm, 440 nm and 220 nm, respectively.
360 nm, 330 nm, 440 nm and 220 nm, respectively.
Effect of concentration on adsorption of phenols on metal ferrocyanides
Adsorption of the 2-NP, 4-NP, 2-AP and 4-AP on metal ferrocyanides as a function of concentration (10-4 to 10-5 M) was studied at a pH of 7.0 ![]() 0.1 and at room temperature 30
0.1 and at room temperature 30 ![]() 1oC. A series of 15 mL test tubes were employed for the adsorption studies. 10 mL of the buffered phenol was placed into test tubes and 25 mg of the metal ferrocyanide was added to each test tube and the tube was stoppered. The test tubes were then agitated for 6 hours until equilibrium was attained. The contents were then centrifuged at 3000 rpm for 15 minutes. The supernatant liquid was decanted and the pH of the solution was again noted on pH meter and it was found to be unchanged. The absorbance of 2-NP, 4-NP, 2-AP and 4-AP solutions were measured spectrophotometrically at their corresponding
1oC. A series of 15 mL test tubes were employed for the adsorption studies. 10 mL of the buffered phenol was placed into test tubes and 25 mg of the metal ferrocyanide was added to each test tube and the tube was stoppered. The test tubes were then agitated for 6 hours until equilibrium was attained. The contents were then centrifuged at 3000 rpm for 15 minutes. The supernatant liquid was decanted and the pH of the solution was again noted on pH meter and it was found to be unchanged. The absorbance of 2-NP, 4-NP, 2-AP and 4-AP solutions were measured spectrophotometrically at their corresponding ![]() 360 nm, 330 nm, 440 nm and 220 nm, respectively. The amounts of phenols adsorbed was calculated by the difference in concentration before and after the adsorption.
360 nm, 330 nm, 440 nm and 220 nm, respectively. The amounts of phenols adsorbed was calculated by the difference in concentration before and after the adsorption.
NOTE
1 Department of Chemistry, Faculty of Natural Sciences, University of Guyana, PO Box 101110, Georgetown, Guyana, brijtew@yahoo.com
2 Department of Chemistry, University of Saskatchewan, 110 Science Place, Saskatoon, SK, 57N 5C9, Canadá
REFERENCES
1. Wade, L. G., "Chemical compound: phenol" Ultímate Reference Suite, Chicago: 2010, Encyclopedia Brítanníca. [ Links ]
2. Santana, C. M, Ferrera, Z. S., Padrón, M. E. T., Rodríguez, J. J. S. 2009, Molecules, 14, 298. [ Links ]
3. Manahan, S., Environmental Chemistry, CRC Press LLC, 7th Ed., 2000, Boca Ratón, U.S.A., pp. 741. [ Links ]
4. Politycka, B., Adamska, D. 2003, Pol J. Environ. Stud., 12(1), 95. [ Links ]
5. Ashraf, H., Husain, Q. 2009, Biotechnol. Bioprocess Eng., 14, 536. [ Links ]
6. Lee, Y., Yoon, J., Gunten, J. 2005, Appl. Chem., 9(1), 205. [ Links ]
7. Qadeer, R, Rehan, A. H. 2002, Turk. J. Chem., 26, 357. [ Links ]
8. Kunin, R 1977, Polym. Eng. Sci, 17(1), 58. [ Links ]
9. Mbui, D. N., Shiundu, P. M, Ndonye, R. M, Kamau, G. M. 2002, J. Envíron. Monit, 4, 978. [ Links ]
10. Martins, R. C. C, Leal, H. M., Quinta - Ferreira, R. M. O., Proceedings oí the World Congress on Engineeríng and Computer Science, 2007, San Francisco. [ Links ]
11. Meikap, M. C, Rot, G. K. 1997, J. IPHE, 3, 54. [ Links ]
12. Moraitopoulos, I., Ioannou, Z. Simitzis, J. 2009, World Acad. Scí. Eng.Technol, 58, 218. [ Links ]
13. Flores, J. C, Owen, T. C, Raulin, F., (eds.), First Steps in the Origin of Life in the Universe, Kluwer Academic Publishers, 2001, Dordrecht, pp. 59. [ Links ]
14. Ali, S. R., Mam, T. 2004, Kamaluddin, Astrobiology, 4 (4), 420. [ Links ]
15. Tewari, B. B., Kamaluddin, M, D. 1998, Colloíds and Surfaces A: Physicochem. Eng. Aspects, 131, 89. [ Links ]
16. Fenchel, T., The Origin and Eariy Evolutíon of Lífe, Oxford University Press, 2002, Oxford, pp. 14. [ Links ]
17. Kamaluddin, M.D., Deopujari, S., Singh, W. M. 1986, Origins of Life and Evol Biosphere, 16(3-4), 496. [ Links ]
18. Banin, A., Lawless, J. G., Mazzurco, J., Church, F. M, Margulies, L. Orenberg, A. B. 1985, Origins of Life, 15, 89. [ Links ]
19. Kamaluddin M.D., Nath, M, Deopujari, S. W. Sharma, A. 1990, Origins of Life Evol Biosphere, 20, 259. [ Links ]
20. Tewari, B. B, Kamaluddin M.D. 1997, J. Colloíd Interface Scí, 193, 167. [ Links ]
21. Griffith, W. P. 1962, Q. Rev. (London), 16, 188. [ Links ]
22. Garg, H. B., Beach, N. A. 1965, J. Am. Chem. Soc, 87, 3340. [ Links ]
23. Singh, B., Nema, K. P. 2015, Res. J. Chem. Scí. 5(1), 78. [ Links ]
24. Kulkarni, S., Kaware, J. 2013, Int. J. Scí. Eng. Res. (USER), 1(2), 88. [ Links ]
25. Adam, O. A, Al-Dujaili, A. H. 2013, J. Chem., Hindawi, 1, 1 (article ID 694029). [ Links ]
26. Djebbar, M., Djafri, F., Bouchekara, M., Djafri, A. 2012, Appl. Water Sel, 2 (2), 77. [ Links ]
27. Loncar, N., Bozic, N., Andelkovic, I., Milovanovic, A, Dojnov, B., Vujcic, M., Roglic, G., Vujcic, Z. 2011, J. Serh. Chem. Soc, 76(4), 513. [ Links ]
28. Saravankumar, K., Kumar A. 2013, Afr. J. Agri. Res., 8(23), 2965 [ Links ]
29. Girish, C. R., Murty, V. R 2014, Int. ScholaryRes. Notices, Hindawi, 1, 1 (article ID 201626). [ Links ]
30. Lathasree, S. 2015, J. Chem. Pharma. Res., 7(3), 1833. [ Links ]
31. Yousaf, M. M., Ibrahim, M.M. 2014, Pak. J. Chem., 4(1), 31. [ Links ]
32. Girish, C. R., Murty, V. R 2012, J. Environ. Res. Develop., 6(3A), 763. [ Links ]
33. Pradhan, B. K. Sandle, N. K. 1999, Carbón, 37, 689. [ Links ]
34. Langmuir, I. 1918, J. Am. Chem. Soc, 40, 1361. [ Links ]
35. Kourim, V., Rais, J., Million, B. 1964, J. Inorg. Nucí. Chem., 26,1111. [ Links ]