Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Boliviana de Química
On-line version ISSN 0250-5460
Rev. Bol. Quim vol.33 no.1 La Paz Apr. 2016
LOS COMENTARIOS Y / O ARTÍCULOS TEÓRICOS
Synthesis of alkenes: claisen rearrangement of allyl vinyl ethers, part i; mechanistic views; the organic chemistry notebook series, a didactical approach, N2 9
José A. Bravo1,*, José L. Vila2
*Corresponding author: joseabravo@outlook.com
Abstract
We present now the ninth theoretical assay in the series: "The Organic Chemistry Notebook Series, a Didactical Approach".
The aim of this series of studies is to help students to have a graphical view of organic synthesis reactions of diverse nature. In the present chapter, we board the fascinating thematic on Claisen rearrangements of ally-vinyl ethers as a way to obtain alkenes. Starting from allyl alcohols by rearrangements of the corresponding derivative allyl vinyl ether it is feasible the obtaining of carbonyl compounds unsaturated at the positions: yS; these include aldehydes, ketones, esters and amides. We have assayed theoretical mechanisms for the conversion of allyl alcohol into allyl vinyl ether. The synthesis of the insect pheromone: 3,7-dimethyl-2,6-decadiene-1,10-diol from allyl alcohol and methyl orthoacetate is mechanistically reviewed. The formation of j^unsaturated N,N-dimethyl amides is mechanistically discussed. To end the part I of this series on synthesis of alkenes by Claisen rearrangement of allyl vinyl ethers, we study a feasible mechanism of an alternative to the acidic and heated process involved in the synthesis of the j#unsaturated carbonyl compounds: the Claisen rearrangement of lithium enolates, or the trimethyl or the t-butyl dimethylsilyl enol ethers. We have taken a series of reactions reviewed by W. Carruthers in 'Some modern methods of organic synthesis', and we have proposed didactical and mechanistic views for them. This theme is included in the chapter "Formation of carbon-carbon double bonds" in the mentioned text.
Keywords: Organic Chemistry, Alkenes, Allyl vinyl ethers, Claisen rearrangment, Mechanisms of Reactions, W. Carruthers.
Resumen
Spanish title: Síntesis de alquenos mediante transposición de Claisen de éteres alil-vinílicos, parte I; vistas mecanicísticas; de la serie: El cuaderno de notas de química orgánica, un enfoque didáctico, N"9.
Llegamos al noveno ensayo teórico en la serie: "El cuaderno de química orgánica, un enfoque didáctico". El objetivo de esta serie de estudios es ayudar a los estudiantes a disponer de una visión gráfica de reacciones de síntesis orgánicas de diversa naturaleza. En el actual capítulo, abordamos el fascinante tema de la transposición de Claisen de éteres alil-vinílicos como un método de obtener alquenos. A partir de alcoholes alílicos y por transposición del correspondiente derivado, alil-vinil éter, es factible la obtención de compuestos carbonílicos insaturados en posición: y8; éstos incluyen aldehídos, cetonas, esteres y amidas. Hemos propuesto mecanismos teóricos para la conversión de alcohol alílico en éter alil-vinílico. La síntesis de la feromona de insectos: 3,7-dimetil-2,6-decadien-1,10-diol a partir de alcohol alílico y ortoacetato de metilo, es mecanicísticamente revisada. Se discute también por mecanismos, la formación de N,N-dimetil amidas j#insaturadas. Para terminar la parte I de esta serie de síntesis de alquenos por transposición de Claisen de éteres alil-vinílicos, hemos estudiado un mecanismo factible, alternativa sintética al proceso acídico y bajo calor involucrado en la síntesis de compuestos carbonílicos j^insaturados: la transposición de Claisen de enolatos de litio, ó trimetíl ó terbutil dimetilsilil enol éteres. Hemos tomado una serie de reacciones revisadas por W. Carruthers en: 'Some modern methods of organic synthesis', para las cuales hemos propuesto vistas mecanicísticas y didácticas. Este tema esta incluido en el capítulo "Formation of carbon-carbon double bonds" del mencionado texto.
INTRODUCTION
During master classes of organic chemistry we noticed that students are confronted with a lack of knowledge with regard to mechanisms. For instance, oxidation-reduction reactions which are among the most commonly employed constitute a kind of black box for the student's mind. A mechanistic approach about any kind of reaction enhances the capacity of facing new reactions with respect to an understanding of all processes involved in them, and also develops synthetic creativity. As academics we feel concerned with the didactical importance of covering these needs in debutant students in organic synthesis. This, the synthesis of alkenes by Claisen rearrangement of allyl vinyl ethers, part I; mechanistic views; is the ninth study in the series: "The Organic Chemistry Notebook Series, a Didactical Approach" [1-8].
REACTIONS AND THEIR MECHANISTIC PROPOSALS, DISCUSSION
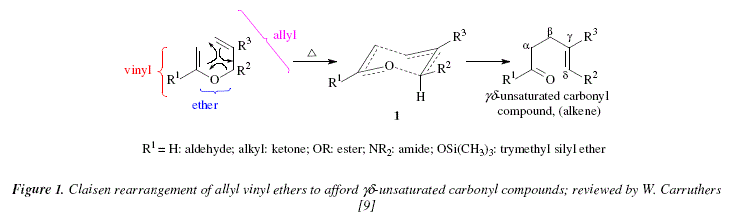
The Claisen rearrangement of allyl vinyl ethers constitutes a stereoselective way to the production of ![]() unsaturated aldehydes, as well as ketones, esters and amides [9]. The starting point of theses syntheses is the allyl alcohol. A number of natural products have been synthesized by these means [9-12]. The Claisen reaction comprises a [3,3]-sigmatropic rearrangement [9]. The mechanism is considered as concerted through a transition state that involves a six-memeberd cycle ([9], Fig. 1).
unsaturated aldehydes, as well as ketones, esters and amides [9]. The starting point of theses syntheses is the allyl alcohol. A number of natural products have been synthesized by these means [9-12]. The Claisen reaction comprises a [3,3]-sigmatropic rearrangement [9]. The mechanism is considered as concerted through a transition state that involves a six-memeberd cycle ([9], Fig. 1).
The reaction gives the formation of a new ![]() bond and a new cr bond. The synthetic value of this reaction lays in the fact that it is highly stereoselective, especially when
bond and a new cr bond. The synthetic value of this reaction lays in the fact that it is highly stereoselective, especially when ![]() [9]. The preferred stereoisomer is the E-configured regarding the new
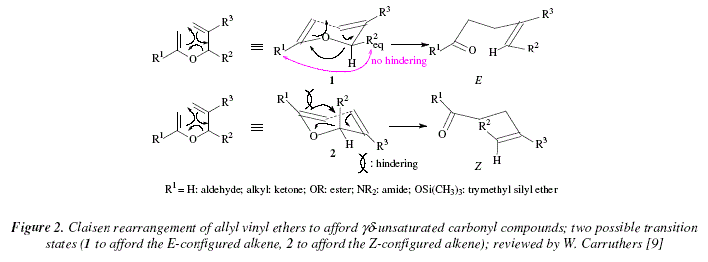
[9]. The preferred stereoisomer is the E-configured regarding the new ![]() bond formed [9]. It also gives a preferred stereochemical disposition of the substituents on the single bond [9]. In Fig. 1, the chair conformer (1) is more adequate for the cyclic transition state [9]. In this, substituent R2 occupies the equatorial disposition and there is no hindering between R1 and R2eq. Fig. 2 shows the results of the cyclisation for the formation of both stereoisomers: E (chair conformer [1], R2eq, no hindering between R1 and R2eq), and Z (inversed chair conformer [2], R2ax, hindering between R1 and R2ax) [9].
bond formed [9]. It also gives a preferred stereochemical disposition of the substituents on the single bond [9]. In Fig. 1, the chair conformer (1) is more adequate for the cyclic transition state [9]. In this, substituent R2 occupies the equatorial disposition and there is no hindering between R1 and R2eq. Fig. 2 shows the results of the cyclisation for the formation of both stereoisomers: E (chair conformer [1], R2eq, no hindering between R1 and R2eq), and Z (inversed chair conformer [2], R2ax, hindering between R1 and R2ax) [9].
The Claisen cyclisation uses allyl vinyl ethers prepared from allyl alcohols in an acid catalyzed reaction that involves an ether exchange [9]. Figure 3 shows the allyl alcohol 3 condensed with ethyl vinyl ether in a reaction catalyzed by the Lewis acid Hg2+ (+ 2AcO-), the adduct is the allyl vinyl ether [9]. The subsequent pyrolysis provokes the Claisen cyclisation to afford the alkene-aldehyde 4 [9]. Figure 4 shows an explicit mechanistic view for this reaction.
Comments
Figure 4 shows the allyl alcohol conversion into allyl vinyl ether where the acid catalyst is Hg2+. This Lewis acid interacts with electron rich centers as oxygen, both, in the substrate (allyl alcohol) and in the ethyl vinyl ether reagent. Also, mercury interacts with the double bond in ethyl vinyl ether. The cation Hg2+ is placed between both compounds. This provokes unequally, the increasing of the electrophilic character of the carbon that sustains the oxygen atom in both molecules; the more electrophilic suffers the nucleophilic attack from the oxygen of the other compound, namely, the allyl alcohol oxygen attacks the oxyvinyl carbon and dispatches thus an ethoxide and generates the allyl vinyl ether protonated. The nucleophilic attack on the oxygenated vinyl carbon is preferred over the attack on the oxygenated methylene carbon of the ethyl moiety of the ethyl vinyl ether. This is due to an intermediate that gives more stability to the global reaction when the oxygen electrons displace the two ![]() electrons of the double bond over the extreme carbon of the vinyl. The reestablishment of the double bond occurs with elimination of the ethoxide from the ethyl vinyl ether. The attack over the ethyl moiety of the ether is unfeasible because (in contrast with the attack over the vinyl moiety, where there is a mobile electron pair [
electrons of the double bond over the extreme carbon of the vinyl. The reestablishment of the double bond occurs with elimination of the ethoxide from the ethyl vinyl ether. The attack over the ethyl moiety of the ether is unfeasible because (in contrast with the attack over the vinyl moiety, where there is a mobile electron pair [![]() bond]), it would be necessary the breaking of a crbond
bond]), it would be necessary the breaking of a crbond ![]() instead of the more easily breakable
instead of the more easily breakable ![]() bond of the vinyl moiety. The following is the Claisen cyclisation that affords to the j^unsaturated aldehyde (3).
bond of the vinyl moiety. The following is the Claisen cyclisation that affords to the j^unsaturated aldehyde (3).
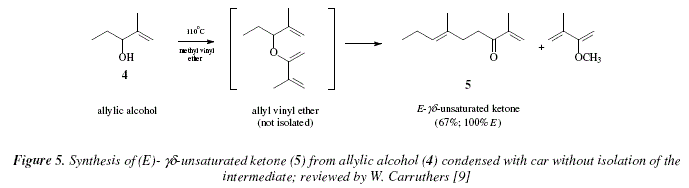
The use of vinyl ethers with different substituents make possible the obtaining of ![]() unsaturated ketones [9]. For example, the allyl alcohol 4 by condensation with 2-methoxy-3-methyl-1,3-butadiene afforded the E-
unsaturated ketones [9]. For example, the allyl alcohol 4 by condensation with 2-methoxy-3-methyl-1,3-butadiene afforded the E-![]() unsaturated ketone 5 [9]. The reaction passes directly over the intermediate allyl-vinyl ether which is not isolated [9]. The presence of the isomer Z has not been detected [9,13], see Fig. 5; and see Fig. 6 for a mechanistic explanation.
unsaturated ketone 5 [9]. The reaction passes directly over the intermediate allyl-vinyl ether which is not isolated [9]. The presence of the isomer Z has not been detected [9,13], see Fig. 5; and see Fig. 6 for a mechanistic explanation.
Comments
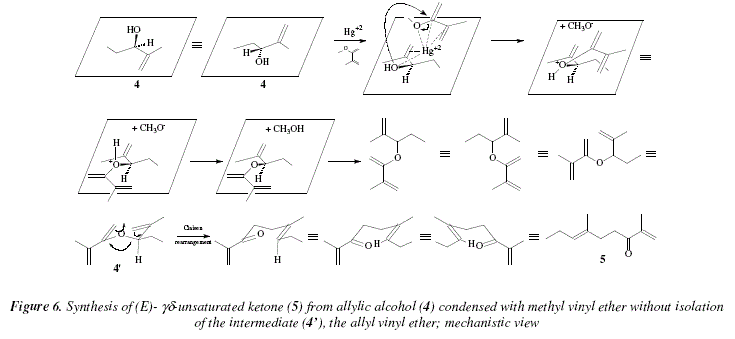
In Fig. 6, and as mentioned before, the cation Hg2+ interacts in the interplanar region, between the allyl alcohol and the methyl vinyl ether. This acid-base interaction exacerbates the electrophilic character of the carbon that sustains the methoxy group of the methyl vinyl ether provoking the nucleophilic attack of the oxygen of the allylic alcohol to replace the methoxy group of the methyl vinyl ether. The elimination of methanol generates the adduct that arranges in the chair conformation, namely the transition state 4' that subsequently conducts to the Claisen rearrangement to afford the E-![]() unsaturated ketone 5.
unsaturated ketone 5.
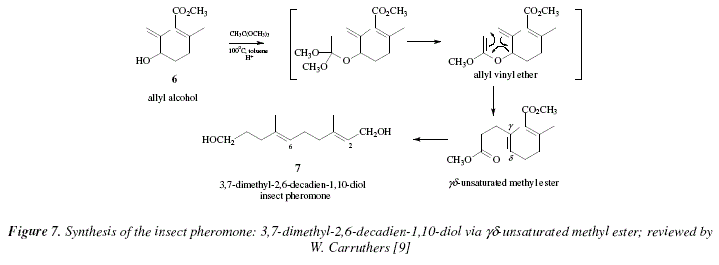
Another illustrative example is the obtaining of the insect pheromone 7 (3,7-dimethyl-2,6-decadien-1,10-diol [14]) using the allyl alcohol 6 and the methyl orthoacetate [9]. The reaction is catalyzed by acid as well; a weak acid was used (propionic acid ) [9]. The product is a ![]() unsaturated ester [9]. See Fig. 7. In a first part, a mixed orthoester is formed, and methanol is dispatched to afford a ketene acetal. This is the substrate that by cyclisation gives the
unsaturated ester [9]. See Fig. 7. In a first part, a mixed orthoester is formed, and methanol is dispatched to afford a ketene acetal. This is the substrate that by cyclisation gives the![]() unsaturated ester [9]. See Fig. 8 for a mechanistic approach. As information let us mention that
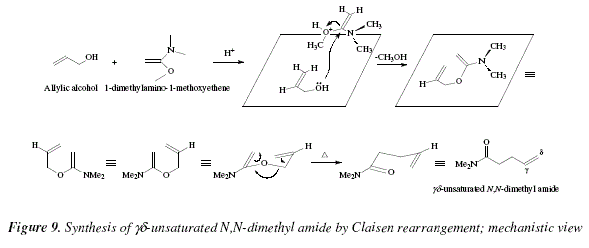
unsaturated ester [9]. See Fig. 8 for a mechanistic approach. As information let us mention that ![]() unsaturated N,N-dimethyl amides can be obtained in a similar manner using allyl alcohols with 1-dimethylamino-1-methoxyethene, or its precursor N,N-dimethylacetamide dimethyl acetal [9,15]. See Fig. 9 for a mechanistic view of this reaction.
unsaturated N,N-dimethyl amides can be obtained in a similar manner using allyl alcohols with 1-dimethylamino-1-methoxyethene, or its precursor N,N-dimethylacetamide dimethyl acetal [9,15]. See Fig. 9 for a mechanistic view of this reaction.
Comments
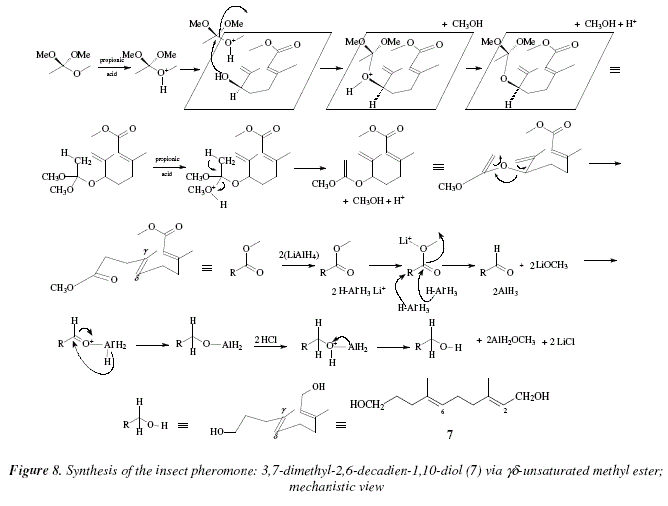
In Fig. 8, the substrate orthoester is catalyzed by proton from propionic acid, towards the nucleophilic attack of the allylic alcohol oxygen, eliminating thus, methanol from substrate. The resulting protonated adduct resturns the proton to the catalyst. The mixed orthoester resulting, receives a proton from the catalyst (propionic acid). The protonated species eliminates methanol to form an alkene (a ketene acetal). This intermediate (not isolable) rearranges to form the ![]() unsaturated ester. The diester is reduced until diol by two equivalents of AlLiH4. These procedures present the disadvantage of a limited number of substrates, due to the high temperature and the acid medium employed, being not suitable for compounds sensitive to such conditions [9].
unsaturated ester. The diester is reduced until diol by two equivalents of AlLiH4. These procedures present the disadvantage of a limited number of substrates, due to the high temperature and the acid medium employed, being not suitable for compounds sensitive to such conditions [9].
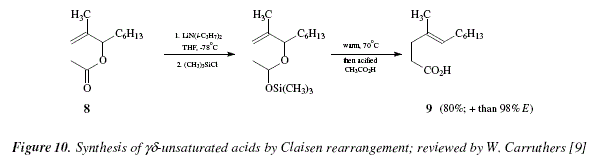
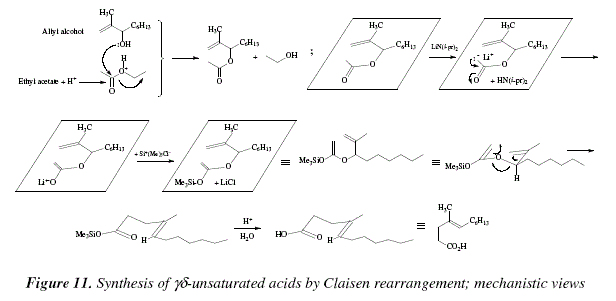
For compounds containing functional groups susceptible to pirolysis or acid, it is feasible the transformation of lithium enolates via Claisen rearrangement to form ![]() unsaturated acids [9]. An alternative is the use of trimethylsilyl or t-butyldimethylsilyl enol ethers [9]. The unsaturated acids are obtained by hydrolysis of the trimethylsilyl esters in high yields; the reaction conditions are mild and softly alkaline. The major compound obtained is the E isomer [9,16]. The allyl acetate 8, was transformed in the
unsaturated acids [9]. An alternative is the use of trimethylsilyl or t-butyldimethylsilyl enol ethers [9]. The unsaturated acids are obtained by hydrolysis of the trimethylsilyl esters in high yields; the reaction conditions are mild and softly alkaline. The major compound obtained is the E isomer [9,16]. The allyl acetate 8, was transformed in the ![]() unsaturated acid 9 (80% yield, almost fully E-selectivity), see Fig. 10 for the global reaction, and see Fig. 11 for a mechanictic proposed view.
unsaturated acid 9 (80% yield, almost fully E-selectivity), see Fig. 10 for the global reaction, and see Fig. 11 for a mechanictic proposed view.
Comments
In the present case the prime matter is an ester coming from the condensation of the allyl alcohol and an ester. The allyl alcohol always acts as a nucleophile. In previous examples it was an allyl alcohol who attacked the ethyl vinyl ether, or the methyl vinyl ether. We mentioned another example where the allylic alcohol, through its hydroxyl, attacks an orthoester. Also, the allylic alcohol can attack to 1-dimethylamino-1-methoxyethene. In Fig.11, the allylic alcohol attacks a previously protonated ester to afford a ![]() unsaturated ester. The activation of this substrate is in charge of LiN(i-pr)2, a strong base that deprotonates the methyl of the methyl ester. The generated carbanion transfers its electronic charge to the carbonyl oxygen where it pairs with Li+. A cationic exchange takes place between this Li+ and the cation trimethyl silyl. The allyl vinyl ether to be object of the Claisen rearrangement is ready. Once the cyclisation happens, the oxysilyl is hydrolyzed under acid conditions to afford the j#unsaturated carboxylic acid.
unsaturated ester. The activation of this substrate is in charge of LiN(i-pr)2, a strong base that deprotonates the methyl of the methyl ester. The generated carbanion transfers its electronic charge to the carbonyl oxygen where it pairs with Li+. A cationic exchange takes place between this Li+ and the cation trimethyl silyl. The allyl vinyl ether to be object of the Claisen rearrangement is ready. Once the cyclisation happens, the oxysilyl is hydrolyzed under acid conditions to afford the j#unsaturated carboxylic acid.
ACKNOWLEDGEMENT
The authors express their gratitude to Prof. Eduardo Palenque from the Department of Physics, Universidad Mayor de San Andrés, for his bibliographic support.
NOTES
1 Department of Chemistry, Laboratorio de Fitoquímica, Instituto de Investigaciones en Productos Naturales IIPN, Universidad Mayor de San Andrés UMSA, P.O. Box 303, Calle Andrés Bello s/n, Ciudad Universitaria Cota Cota, Phone 59122792238, La Paz, Bolivia, jabravo@umsa.bo
2Department of Chemistry, Laboratorio de Síntesis y Hemisíntesis, Instituto de Investigaciones en Productos Naturales IIPN, Universidad Mayor de San Andrés UMSA, P.O. Box 303, Calle Andrés Bello s/n, Ciudad Universitaria Cota Cota, Phone 59122795878, La Paz, Bolivia, joselu62@hotmail.com
REFERENCES
1. Bravo, J. 2005, The organic chemistry notebook series, a didactical approach. Theoretical mechanistic approach to diasteroselective synthesis of cis-1,2-dialkenylcyclopropanols and subsequent oxy-Cope rearrangement by Jin Kun Cha et al, Rev. Bol. Quim., 23, 1-10. [ Links ]
2. Bravo, J.A., Mollinedo, P., Peñarrieta, J.M., Vila, J.L. 2013, Mechanistic views of intramolecular hydroxycyclopropanation of ¿y-vinyl carboxylic esters, Rev. Bol. Quim., 30 (1), 24-41. [ Links ]
3. Bravo, J.A., Vila, J.L. 2014, Mechanistic views of stereoselective synthesis of tri and tetra-substituted alkenes, part I; the organic chemistry notebook series, a didactical approach, n° 3. Rev. Bol. Quim., 31 (1), 61-67. [ Links ]
4. Bravo, J.A., Vila, J.L. 2015, Mechanistic views of stereoselective synthesis of tri and tetra-substituted alkenes, part II; the organic chemistry notebook series, a didactical approach, n° 4, Rev. Bol. Quim., 32 (1), 15-23. [ Links ]
5. Vila, J.L., Bravo, J.A. 2015, Synthesis of alkenes by fragmentation reactions; Mechanistic views; the organic chemistry notebook series, a didactical approach, n° 5, Rev. Bol. Quim., 32 (2), 37-44. [ Links ]
6. Bravo, J.A., Vila, J.L. 2015, Synthesis of alkenes by oxidative decarboxylation of carboxylic acids; Mechanistic views; the organic chemistry notebook series, a didactical approach, n° 6, Rev. Bol. Quim., 32 (3), 45-52. [ Links ]
7. Bravo, J.A., Vila, J.L. 2015, Synthesis of alkenes from ketones via arylsulphonyl-hydrazones; mechanistic views; the organic chemistry notebook series, a didactical approach, n° 7, Rev. Bol. Quim., 32 (4), 82-89. [ Links ]
8. Bravo, J.A., Vila, J.L. 2015, Stereospecific synthesis of alkenes from 1,2-diols; mechanistic views; the organic chemistry notebook series, a didactical approach, n° 8, Rev. Bol. Quim., 32 (5), 121-125. [ Links ]
9. Carruthers, W. Some Modern Methods of Organic Synthesis, Cambridge University Press, 3rd ed., 1987, Worcester, U.K., pp. 167-169. [ Links ]
10. Rhoads, S.J., Raulins, N.R. 2011, The Claisen and Cope rearrangements. Organic Reactions. 22, pp.1-252. [ Links ]
11. Ziegler, F.E. 1977, Stereo- and regiochemistry of the Claisen rearrangement: application to natural products synthesis, Acc. Chem. Res., 10, 227-232. [ Links ]
12. Bartlett, P.A., 1980, Stereocontrol in the synthesis of acyclic systems: application to natural product synthesis, Tetrahedron, 36, 2-72. [ Links ]
13. Daub, G.W., Sanchez, M.G., Cromer, R.A., Gibson, L.G. 1982, Regioselectivity of the Ketal Claisen Rearrangement, J. Org. Chem., 47, 743-745. [ Links ]
14. Katzenellenbogen, J.A., Cristy, K.J. 1974, Stereoselectivity of the rearrangement of allylsiloxyvinyl ethers. A highly stereoselective synthesis of a diol found in the pheromonal secretion of the queen butterfly. J. Org. Chem. 39, 3315-3318. [ Links ]
15. Felix, D., Gschwend-Steen, K., Wick, A.E., Eschenmoser, A. 1969, CLAISEN'sche Umlagerungen bei Allyl- und Benzylalkoholen mit 1-Dimethylamino-1-methoxy-äthen, Helv. Chim. Acta, 52 (4), 1030-1042. [ Links ]
16. Ireland, R.E., Mueller, R.H., Willard, A.K. 1976, The ester enolate Claisen rearrangement. Stereochemical control through stereoselective enolate formation, J. Am. Chem. Soc., 98 (10), 2868-2877. [ Links ]