Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Boliviana de Química
On-line version ISSN 0250-5460
Rev. Bol. Quim vol.30 no.1 La Paz 2013
ARTICULO
USE TO FERRATE (VI) A GREEN CHEMICAL FOR THE ENVIRONMENT REMEDIATION
Adonica Henry-Chase, Brij Bhushan Tewari*
Department of Chemistry, University of Guyana, P.O. Box: 101110, Georgetown, Guyana
Keywords: Ferrate (VI) technology, potassium ferrate, bariumferrate, green chemical, wastewater treatment.
ABSTRACT
The ferrate (VI) a green chemical due its novel properties such a oxidizing power, selective reactivity, stability as salt and non-toxic by-products of ferric ion, much progress has been made on its application to water and wastewater treatment as efficient oxidant, coagulant and disinfectant. Potassium and barium ferrate are synthesized, purified and characterized. These two chemicals were used to treatment of water samples collected from Guyana Water Incorporation Georgetown (GWI Georgetown), Guyana Water Incorporation Linden (GWI Linden), Tap water South Ruimveldt (Tap Water SR) and University of Guyana Pond (UG Pond). Potassium ferrate is found to be more effective in water treatment in comparison to barium ferrate.
INTRODUCTION
Iron is typically found as a metal ion +2 [Fe (II)] or +3 [Fe (III)] oxidation states, the presence of hyper - valency states of iron Fe (IV), Fe (V), Fe (VI) have been known in certain environment. Of the hyper - valent iron, only ferrate Fe (VI), +6 oxidation states of iron has been known to be obtained in the form of stable salts [1-3]. It has been well documented [4] the potassium ferrate K2Fe04 is a strong and environmental friendly oxidant under the acidic conditions, the redox potential of ferrate (VI) ions (2.2 V) is higher that of molecular ozone (2.0 V)
Fe(VI) O4 2- + 3e- + 8H+ ------> Fe3+ + 4H2O E°= 2.20 V
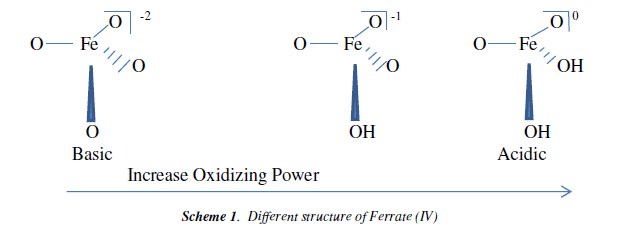
The product of the ferrate (IV) oxidation reaction is considered to be ferric hydroxide, which is useful coagulant and well enhance the water remediation performance. Ferrate (VI) is relatively stable in basic solutions but is very unstable in neutral and acidic solutions. When protons are attached to the ferrate (VI) ion as it goes from basic to acidic solutions, super iron becomes an even more powerful oxidizing agent. Different structure of ferrate with its increasing oxidizing power is shown in Scheme 1.
A search of literature indicated some applications of ferrate viz. oxidize synthetic organic matter [5], remove colour [6], remove cyanide [7], killing bacteria [8] a multifunctional material for treating contaminated water and waste water [9-18]. The Scheme 2 shows some applications of ferrate (VI) to various fields.
The aim of this paper is to
i. Synthesis and characterize potassium and barium ferrates.
ii. Collection of water and wastewater samples from different location of the country (Guyana),
iii. Analyze the collected samples for total hardness, alkalinity, turbidity, colour, iron, chloride, and pH before and after treatment.
iv. Water samples collected from selected región of the country due to importance of the need for the clean and safer water. Each sample location is unique the standpipes and effluents where due for understanding the environment.
RESULTS AND DISCUSSION
Colour test
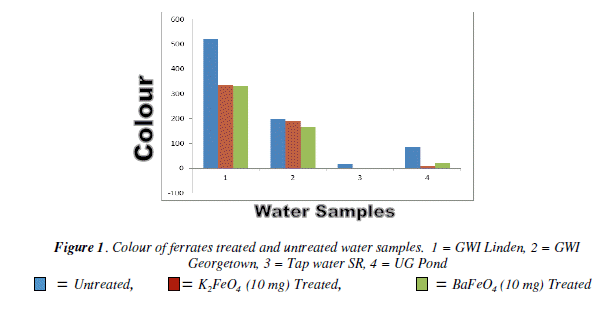
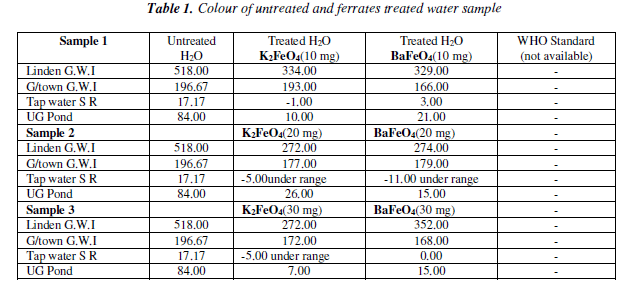
The colour test there was a gradual decrease for both ferrates. When compared to the untreated water the tap water at 20 mg for both ferrates had the lowest result. However, the result was the same at the 30 mg for the potassium ferrate, while the barium ferrate increased. The pond water sample had it lowest result at 30 mg for the potassium ferrate, while the barium ferrate 30 mg and 20 mg were the same. It was the 20 mg mass for both ferrates that had the greatest effect. Bar Chart of the colour test for untreated and potassium and barium ferrates treated contaminated water samples are shown in Figure 1 and valúes are given in Table 1.
pH test
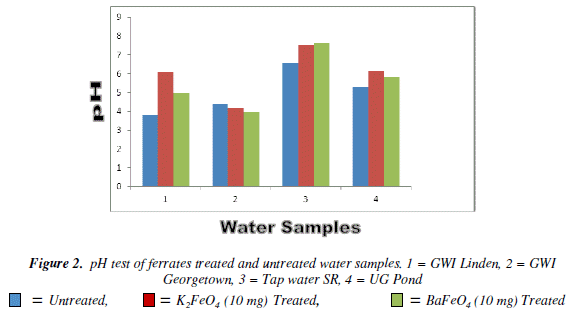
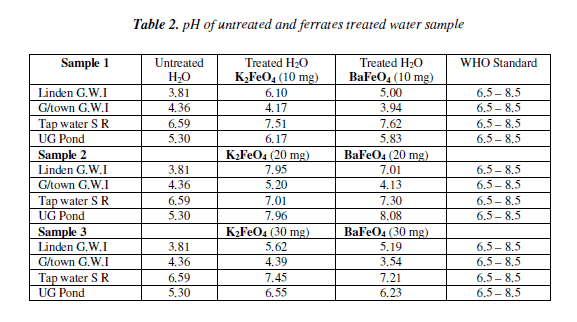
For the pH test the 20 mg mass for both ferrates was more effective, than the other masses. The Georgetown G.W.I. had a gradual increase as the masses were increased, but still it was not in the enough to reach the W.H.O. standards. The most acidic sample was the Linden G.W.I. the potassium ferrate had greater effect than the barium ferrate. The result from the20 mg mass except the Georgetown G.W.I. was less than the W.H.O. standard. However, at the 30 mg mass only the tap and pond water for both ferrates were in the range of the W.H.O. standard while the other sample fall out of range. Bar Chart of pH test for untreated and potassium and barium ferrates treated contaminated water sample is shown in Figure 2 and valúes are given in Table 2.
Turbidity test
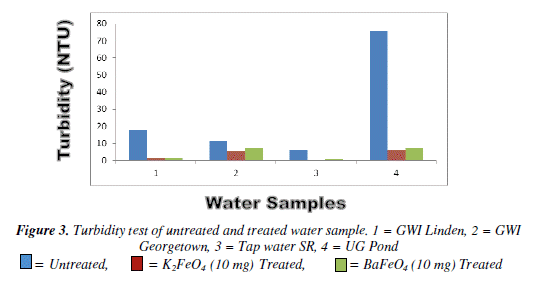
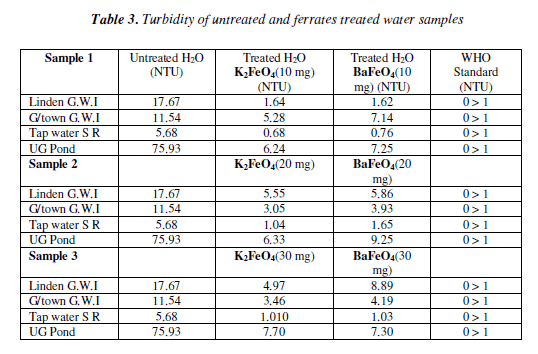
The turbidity test both of ferrates was quite effective. As the masses were increased the turbidity for all of the water samples decreased significantly when compared to the untreated water samples. However, when compared to the World Health Organisation (WHO) most of the samples were out of range. The cause of this could be due to the presence of impurities that might have being present or the time of treatment may ha ve been too short. The 10 mg mass of the potassium ferrate was more effective than that of barium ferrate since most of the water sample treated with the potassium ferrate carne very cióse or within range of the W.H.O. standard.
Bar Chart of turbidity test for untreated and potassium and barium ferrates treated contaminated water sample is shown in Figure 3 and valúes are given in Table 3.
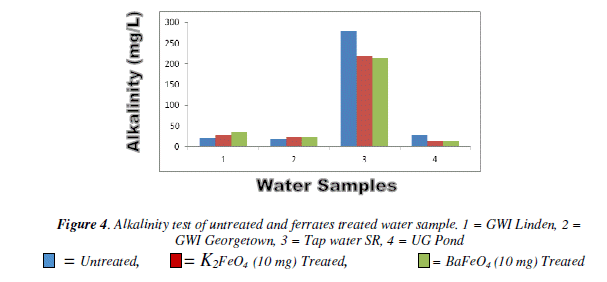
Alkalinity test
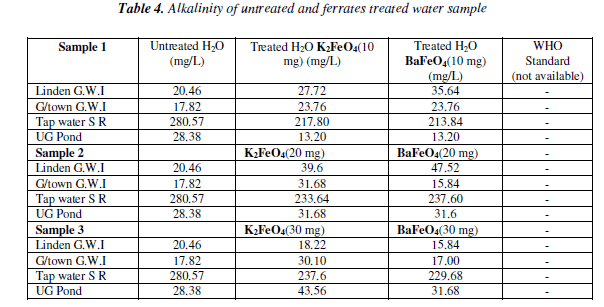
For the alkalinity test the result varied among the different masses and the water sample. However, it was the tap water result for both ferrates that had the lower result than the of the untreated water sample. The pond and tap water for both ferrates had an increased as the mass increased. The potassium ferrate showed a gradual increase, while the barium ferrate fluctuated. Bar chart of alkalinity test for untreated and potassium and barium ferrates treated contaminate water sample is shown in Figure 4 and valúes are given in Table 4.
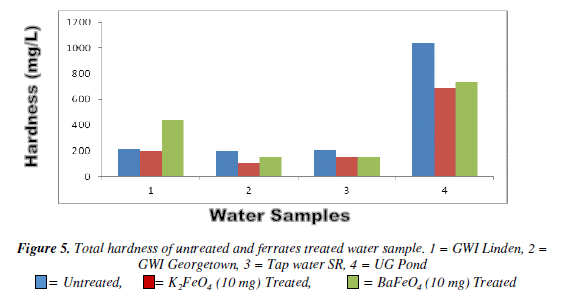
Total Hardness test
For the alkalinity test the result varied among the different masses and the water sample. However, it was the tap water result for both ferrates that had the lower result than the of the untreated water sample. The pond and tap water for both ferrates had an increased as the mass increased. The potassium ferrate showed a gradual increase, while the barium ferrate fluctuated. The total hardness test it was observed that the 10 mg mass for both ferrates was more effective than the other masses. But the potassium ferrate at the 10 mg had a better result than the barium ferrate at 10 mg, because it had the lower result. Though the barium ferrate was not as low, its result was still lower than the untreated water. At the 30 mg mass for both ferrates recorded the highest amount of hardness with the exception of the barium ferrate pond water, which had a similar result to the 10 mg barium ferrate. Bar Chart of the total hardness test for untreated and potassium and barium ferrates treated contaminated water sample is shown in Figure 5 and valúes are given in Table 5.
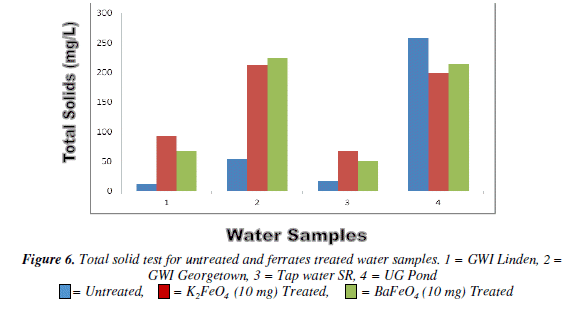
Total Solid test
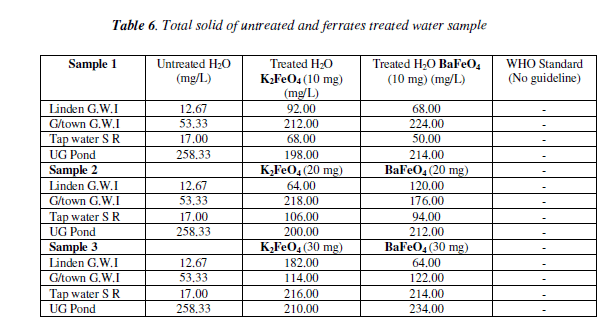
The total solid test there was an overall increased for both of the ferrate as the mass increase, when compared to the untreated water ampie. But pond water had a decreased as the masses were increased. Tap water was the only one with a gradual increase as the mass increase the others fluctuated. However, at the lOmg mass for both ferrates though they were high they were still lower than the untreated water sample.
Bar chart of total solid test for untreated and potassium and barium ferrates treated contaminated water sample in shown in Figure 6 and valúes are given in Table 6.
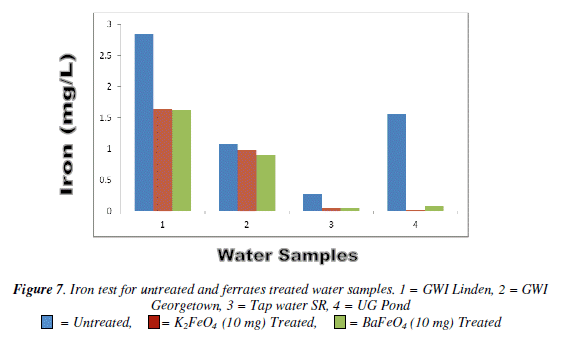
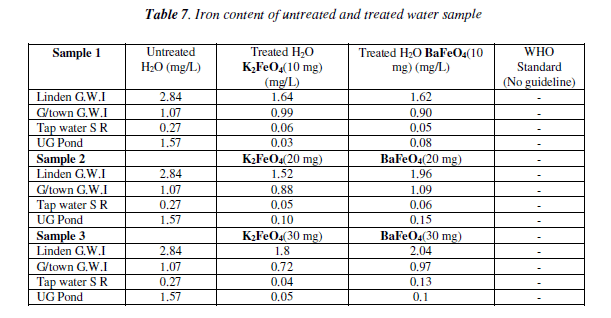
Iron test
With the iron test there was not an overall decrease, but there was a gradual decrease as the mass increased for the Georgetown G.W.I. (Lamaha Canal) and tap water. The Linden G.W.I. barium ferrate had a gradual increased as the mass increased, while for the potassium ferrate it fluctuated. Like the turbidity the 10 mg mass was the most effective than the other masses. Bar Chart of iron test for untreated and potassium and barium ferrate treated contamination water sample is shown in Figure 7 and valúes are given in Table 7.
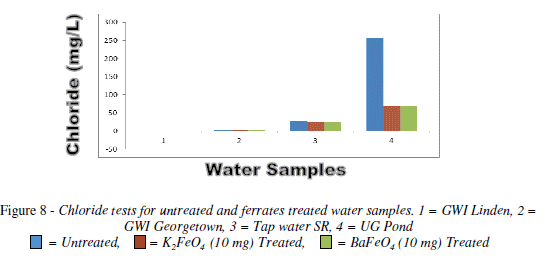
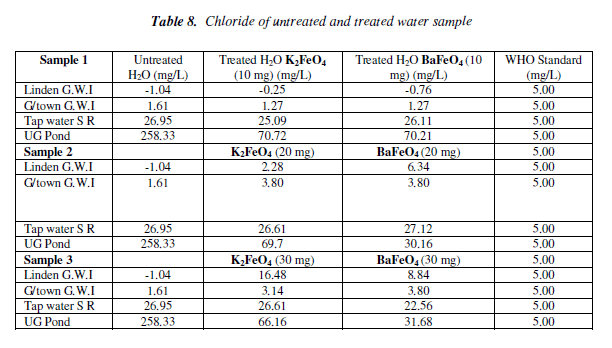
Chloride test
The chloride had an overall increase for both ferrates, with the exception of pond water, which had decreased. The 10 mg mass was the most effective than the other masses. But of the two ferrates treated water it was the potassium ferrate, since most of it results was lower than the W.H.O. standard. The pond water though it was not lower than the W.H.O. standard it decreased as the mass was increased. On the other hand tap water increased as the mass increased for the potassium ferrate but for the barium ferrate it fluctuated. Bar chart of chloride test for untreated and potassium and barium ferrates treated contaminated water sample is shown in Figure 8 and valúes are given in Table 8.
EXPERIMENTAL
Ferrate (VI) can be produced by dry and wet synthetic methods [19, 20] . Dry synthetic method are usually performed using a thermal technique, whereas chemical and electrochemical techniques are applied in wet method. in the present work wet method applied to synthesize ferrate (VI).
Materials
Ferric chloride, sodium hypochloride, sodium hydroxide, ferric nitrate, potassium hydroxide, p otassiium permagnate, barium hydroxide, hexane, diethylether, petroleum sprit, ethanol, etc.
Synthesis of ferrate (VI)
Potassium and barium ferrate are prepared according to the method reported in literature [21] Potassium ferrate was prepared by bubbling chlorine gas generated from concentrated HC1 and KMnO4 through 500 mL of 0.1 M potassium hydroxide. This was done by 5 minutes 15 mL of 0.1 M ferric nitrate was added to 60 mL of KC1O with constant stirring. Solution was filtered and crystals were washed 5 times with 20 mL of hexane and diethylether (1:1) mixture. Crystals were stored in a desiccator.
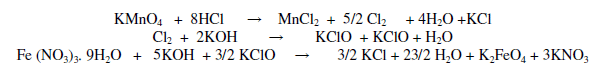
Barium ferrate (BaFeO4) was prepared by adding 5 mg of barium oxide to mixture of 60 mL of 0.1 M NaClO and 15 mL of 0.1 M ferric nitrate at room temperature (29+ 1°C) [22]. Precipítate was filtered and washed with 20 mL of hexane and diethyl ether mixture (1:1) three times to remove water and other impurities.
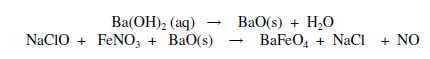
The reactions of both ferrates are exothermic and vigorous with a very strong chlorine odor especially the barium ferrate formation.
Purification offerrates
Ferrates are purification by literature method [3]. Ferrate was washed with 13 mL of petroleum sprit. It further washed with 3 to 5 times with 20 mL of 95 % ethanol. Precipítate was further washed with petroleum sprit. It further washed with 3 to 5 times, 20 mL of 95 % ethanol. Stirring for 20 minutes, this washing is repeated 3 times. Precipítate is dry and stored in a desiccator.
Characterization of ferrate (VI)
Potassium and barium ferrates are found to have purple black and reddish - brown colour respectively. Visible spectra of ferrate (VI) prepared in this work demónstrate typical ferrate (VI) characteristics absorption 505 nm and 790 nm which is consistent with literature [23,24]. Concentration of ferrate (VI) was determined by titration against bromine oxide. Ferrate solution is prepared in arsenic trioxide. Methyl orange used as an indicator, a yellow colour appeared at the end point.
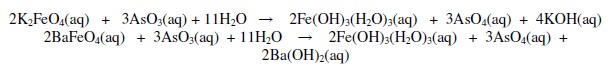
Infrared absorption peak far potassium ferrate was observed at 800 cm_1 (807 cm_1) where as for barium ferrate was observed at 871, 810, 786 cm_1 (870, 812, 780 cm_1). The literature valúes are provided in the bracket [21].
Collection of water samples
Water samples are collected in 500 mL stopper bottles from four different location of the country (Guyana). The order of the water sampling is as follows:
1. Guyana Water Incorporation Linden (GWI Linden)
2. Guyana Water Incorporation Georgetown (GWI Georgetown)
3. Tap water from South Ruimveldt (Tap Water SR)
4. University of Guyana Pond (UG Pond)
Methods for testing water samples
Water sample are tested before and after the addition of ferrates. The turbidity test was done using the turbidity meter at environmental laboratory University of Guyana. It was calibrated with standard range from 0.01 to 800 Nephelometric Turbidity Units (NTU). After which 15 mL of the sample was poured in the sample vial and read off. The iron and colour test were done at the Guyana Water Inc. laboratory, using the portable data login spectrophotometer at wave lengths 510 nm and 120 nm, respectively. A sample of 10 mL was used for the iron test, 5 mL for zeroing the meter; the indicator phenanthroline was added to the 5 mL then read off. For the colour test 25 mL of the sample was used after being calibrated with 25 mL of distilled water. The iron is measured in mg/L, while colour was measured on uints PtCo Alpha. The alkalinity, chloride and total hardness were all done via titration method methods. The alkalinity was using 0.02 N of H2SO4 with methyl orange as it indicator and 25 mL of the sample was used. The end point was noted by a yellow solution. The chloride test was done using 0.143 M of silver nitrate solution, with potassium enrómate as it indicator. A 100 mL of sample was used; the end point was denoted by an orange colour solution. The total hardness was determined by titrating against disodium ethylene diamine tetra-acetic acid (Na2H2 EDTA). A 100 mL of the sample was used 1 mL of pH 10.0 buffer of Erichrome black T-indicator. Green colour appeared at the end point. The total solids test was done by heating, cooling and weighting. A 100 mL sample was placed in an evaporation dish in a sand bath until total evaporation has oceurred, then to the oven, thereafter then cool and weighted.
Tables

NOTAS
*Corresponding author: briitew@vahoo.com
REFERENCES
1. AUDETTE, R J., QUIL, J.W, Inorganic Chemistry, 1972,11,1904 [ Links ]
2. BIEKSI, B.H.J., Free Rod. Res. Comms., 1991,12, 469 [ Links ]
3. THOMPSON, G.W. OCKERMAN, L.T., SCREYER, J.M., J.Am. Chem. Soc, 1951, 73, 1379. [ Links ]
4. JIANG, J.Q., LLOYD, B., Water Res., 2002, 36, 1397. [ Links ]
5. WAITE, T.D., GILBERT, M, J. Wat. Pollut. Control. Fed., 1978, 50, 543. [ Links ]
6. JIANG, J.Q., WANG, S., Environ. Eng. Sci., 2003, 20, 627. [ Links ]
7. SHARMA, V.K., RENDAN, R.A., MILLER, F.J., Alistr. Pap. Am. Chem. Soc, 1999, 217, 110. [ Links ]
8. MURMANN, R.K., Robinson, P. R., Wat. Res. 1974, 8, 543. [ Links ]
9. AUDETTE, R.J., QUIL, J.W, SMITH P.J., Tetrahedron Lett., 1971, 3, 279. [ Links ]
10. BARTZATT, R., TABATABAI, A., CARR, J, Synth. React. Inorg. Met-Org. Chem, 1985,15(a), 1171. [ Links ]
11. KAJAMA, R, Wat. Sci. Technol, 1995, 31, 165. [ Links ]
12. KAJAMA, R, FEMS Microbiol. Lett., 1994,118, 345. [ Links ]
13. SHARMA, V.K., BURNETT, C.R., O'CONNOR, D.B., D.Cabelli, Environ. Sci. Technol., 2002, 36, 4183. [ Links ]
14. D. G. Lee, CHEN, T., J.Org. Chem., 1999, 56, 5341. [ Links ]
15. SHARMA, V.K., Adv. Environ. Res. 2002, 6, 143. [ Links ]
16. READ, J. R, BOUCHER, K. D., MEHLMAN, S. A., WATSON, K. J., Inorg. Chem. Acta, 1998, 267, 159. [ Links ]
17. SHARMA, V.K., RIVERA,W., SIMTH, J. Q. BRIEN, B. O., Environ. Sci. Technol., 1998, 32, 2608. [ Links ]
18.THOMAS, G. W., OCKERMAN, L.T., SCREYER, J.M., J.Am. Chem. Soc, 1951, 73, 1379. [ Links ]
19.BOUJEK, K., SCMIDT, M. J. WRAGG, A. A., Coll. Czech. Chem. Commun., 2002, 65, 133 [ Links ] 20.PERFILIEV, Y. D., Russ. J. Inorg. Chem. 2002, 47, 611. [ Links ] 21.LICHT, S., YU, X., Recent Advance in Fe (VI) Synthesis: Ferrates: Synthesis Properties and Applications in Water and Wastewater Treatment (Ed SHARMA, V.K.), ACS Symposium Series 985, 2008, p.2. [ Links ] 22. HERBER, R.H., JOHNSON, D., Inorg. Chem. 1979,18, 2786. [ Links ] 23.GRIFFITH,W. P., J. Chem. Soc. (A) 1966, 1467. [ Links ] 24.CARRINTON, A., SCHONLAND, D., SYMONS, M.C.R., J. Chem. Soc, 1957, 569. [ Links ]