Revista Boliviana de Química
versión On-line ISSN 0250-5460
Rev. Bol. Quim v.29 n.1 La Paz 2012
ARTÍCULO ORIGINAL
POLYOXYGENATED FLAVONOIDS FROM BACCHARIS PENTLANDII
Santiago Tarqui Tarqui, Yonny Flores Segura and Giovanna R. Almanza Vega1
Laboratorio de Bioorgánica, Instituto de Investigaciones Químicas (IIQ), Carrera de Ciencias Químicas, Universidad Mayor de San Andrés, Calle Andrés Bello y Calle 27 Cota Cota, Edificio FCPN, 2° piso, La Paz - Bolivia.
Keywords: Baccharis pentlandii, poly-oxygenated flavonoids, 8-methoxycirsilineol, xanthomicrol, sideritoflavone, Bolivian Medicinal Plants
ABSTRACT
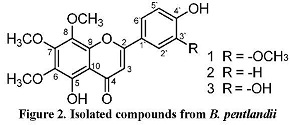
Three highly oxygenated flavonoids were isolated from the leaves of the medicinal plant Baccharis pentlandii commonly known as "Chilca clara" âMayu chilcaâ or simply "Chilca". Flavonoids were identified as: 5,4'dihydroxy-6,7,8,3â-tetramethoxyflavone 8-Methoxycirsilineol (1), 5,4'-dihydroxy-6,7,8-trimethoxyflavone Xanthomicrol (2) and 5,3',4'-trihydroxy-6,7,8-trimethoxyflavone Sideritoflavone (3) by spectroscopic methods. The compounds are the major chemical constituents in the plant and they have proved to be cytotoxic as well as apoptotic substances giving thus the species a potential interest against cancer.
RESUMEN
Tres flavonoides altamente oxigenados fueron aislados a partir de las hojas de la planta medicinal Baccharis pentlandii D.C. comúnmente conocida como "Chilca clara", "Mayu Chilca" o simplemente "Chilca". Los flavonoides fueron identificados como: 5,4'-dihidroxi-6,7,8,3'-tetrametoxiflavona 8-Metoxicirsilineol (1), 5,4'-dihidroxi-6,7,8-trimetoxiflavona Xantomicrol (2) y 5,3',4'-trihidroxi-6,7,8-trimetoxiflavona Sideritoflavona (3) mediante métodos espectroscópicos. Los compuestos identificados son mayoritarios en la planta y tienen antecedentes como citotóxicos y apoptóticos lo cual confiere a la especie un potencial interés contra el cáncer.
INTRODUCTION
The genus Baccharis is considered an important natural resource in Latin America, mainly in the scope of the traditional medicine. Ecological, biological and chemical studies have been done in this genus [1], [2], [3]. Based on their traditional use and great physical availability are considered promising Latin American species for the manufacture of products with value added. The genus Baccharis (Compositae) in America comprises around 400 species of which 20% are used for medicinal purposes [4]. In Bolivia are approximately 60 Baccharis species [5] more of them used in traditional medicine as B. papillosa, B. latifolia, B. pentlandii, B subulata and B. genistilloides. Baccharis pentlandii is a resinous shrub of 0.5 to 2 m tall, with leaves that can grow up to 17 cm long (Figure 1). It is an important medicinal species endemic and characteristic of several regions from Bolivia, Argentina and Peru. In Bolivia is one of the species known as "Chilca" that have medicinal uses, Vidaurre de la Riva [6] mention that the herb known as "Chilca" is identified with the scientific name of Baccharis latifolia in the Market of Medicinal Plants but in the Association of Naturists is identified as Baccharis pentlandii. Reviews of its medicinal use indicate that it is used as an antirheumatic, antiseptic, disinfectant, for pneumonia, cough, sprains, broken bones and dislocations [7].
Pharmacological studies of this species show mainly anti-inflammatory properties. Thus, Abad et. al. [8] showed in its extracts a significant activity against various inflammatory mediators such as COX-1, COX-2 and TNF-a while Gonzales et. al. [9] found that the aqueous extract of B. pentlandii has a moderate acute antiinflammatory activity. The present study shows the isolation of 3 highly oxygenated flavonoids from B. pentlandii: 5,4 '-dihydroxy-6, 7,8,3'-tetramethxyflavone, 8-Methoxycirsilineol (1), 5,4 '-dihydroxy-6,7,8-trimethoxyflavone, Xanthomicrol (2) and 5,4 '3 '-trihydroxy-6,7,8-trimethoxyflavone Sideritoflavone (3). A review of these compounds and other similar compounds in the scientific literature show that they have apoptotic and cytotoxic properties. The compound Xanthocrimol (2), one of the major flavonoids in this species was reported as the main agent with potential cytotoxic
and anti-cancer activity in Dracocephalum kotschyii, a species traditionally used in Iran against cancer [10]. Moreover, the 8-methoxycirsilineol (1) was identified as an effective inhibitor of cytochrome P450 (CYP) [11]. Additionally, several studies of highly oxygenated flavonoids in particular those with multiple methoxy groups have demonstrated their apoptotic properties. For example Igor N. Sergeev searched the apoptotic properties of several 5-hydroxy polimethoxyflavones [12] demonstrating that the hydroxyl group at C-5 is important for the activity, while Sanchez et. al. suggests that the methoxy group at C-8 is responsible for cell death [13]. Therefore, the identification of these major components in B. pentlandii gives it a potential use against cancer.

RESULTS AND DISCUSSIONS
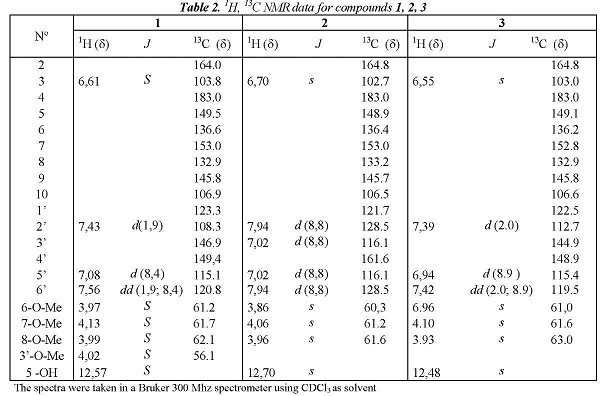
Baccharis pentlandii, a species with high availability in La Paz Valley, was subjected to a study of flavonoids due to the well-known pharmacological importance of these compounds and the traditional use of this plant in Bolivia. Thus, dried and ground leaves were subjected to a process, previously used by our group in the study of other Baccharis species [1,2], to extract non-glycosylated flavonoids and other simple phenolic compounds, which gives an extract called Phenolic Extract of Medium Polarity EFPM, from which we isolated 3 flavonoids. For the elucidation of the isolated compounds we used the following spectra: RMN:H and 13C, :H-COSY, HSQC and HMBC. The RMN13C data obtained and assigned were compared with literature data of the same or similar compounds finally confirming the proposed structures.
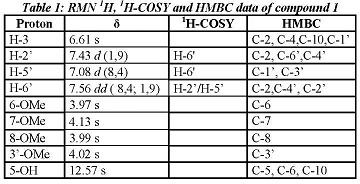
Thus, the :H NMR spectrum of compound 1 has four methyne protons in the aromatic region that corresponds to a spin-spin system meta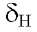 7.43 d (J = 1.9 Hz), orto-meta
7.43 d (J = 1.9 Hz), orto-meta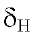 7.56 dd ( J = 8.4 & 1.9 Hz) and orto
7.56 dd ( J = 8.4 & 1.9 Hz) and orto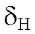 7.8 d (J = 8.4 Hz) from the ring B and the proton H-3 at
7.8 d (J = 8.4 Hz) from the ring B and the proton H-3 at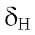 6.61 s. Furthermore, there are four strong signals at
6.61 s. Furthermore, there are four strong signals at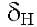 4.13 s, 4.02 s, 3.99 s and 3.97 s (3H), assigned to 4 methoxyl groups and finally shows a characteristic signal at
4.13 s, 4.02 s, 3.99 s and 3.97 s (3H), assigned to 4 methoxyl groups and finally shows a characteristic signal at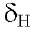 12.57 s assigned to the OH-5 chelated by the carbonyl group at C-4 (Table 1).
12.57 s assigned to the OH-5 chelated by the carbonyl group at C-4 (Table 1).
On the other hand, the RMN13C spectrum shows 19 carbons of which 15 are between 100 and 185 ppm (Table 2) 11 of those are quaternary carbons, one corresponds to a carbonyl group at C-4 (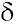 183.0), and 4 are methyne carbons which were assigned based on their direct correlations observed in HSQC.
183.0), and 4 are methyne carbons which were assigned based on their direct correlations observed in HSQC.
The description above suggests a structure of a tetramethoxylated flavone. The locations of the four methoxy substitution were performed under a thorough analysis of its HMBC spectrum. Thus, the methoxy group at C-3' was assigned based on the correlation of the methyl at 4.02 ppm with the carbon C-3' at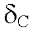 146.9, which also correlates with the H-5 '. For the assignment of the methoxy groups in the ring A we analyzed the correlations of OH-5 with the carbons C-5, C-6, C-10, assigning the methoxy group at 3.97 ppm in C-6. Finally the methoxy groups at C-7 and C-8 were located by their long-range correlations with the carbons at 153.0 and 132.9 ppm respectively, and comparing these data with those obtained by the simulated spectrum obtained in Mestre NOVA program. It is important to mention that there are not good NMR13C data of this compound in the literature since it was first isolated before the 60's when NMR techniques were not well developed. For this the NMR data were compared with those of similar compounds [14], [15].
146.9, which also correlates with the H-5 '. For the assignment of the methoxy groups in the ring A we analyzed the correlations of OH-5 with the carbons C-5, C-6, C-10, assigning the methoxy group at 3.97 ppm in C-6. Finally the methoxy groups at C-7 and C-8 were located by their long-range correlations with the carbons at 153.0 and 132.9 ppm respectively, and comparing these data with those obtained by the simulated spectrum obtained in Mestre NOVA program. It is important to mention that there are not good NMR13C data of this compound in the literature since it was first isolated before the 60's when NMR techniques were not well developed. For this the NMR data were compared with those of similar compounds [14], [15].
Compounds 2 and 3 have similar characteristic signals to the compound 1. On ring A the :H-NMR spectrum shows the OH-5 at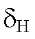 12.70s and
12.70s and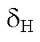 12.48s in 2 and 3 respectively. Positions 6, 7 and 8 are methoxylated showing the methoxy protons correlations with the carbons C-6, C-7 and C-8 respectively, all these data very similar to those of compound 1 (Table 2). Additionally, both compounds showed the characteristic signal of H-3 at
12.48s in 2 and 3 respectively. Positions 6, 7 and 8 are methoxylated showing the methoxy protons correlations with the carbons C-6, C-7 and C-8 respectively, all these data very similar to those of compound 1 (Table 2). Additionally, both compounds showed the characteristic signal of H-3 at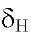 6.70 s for 2 and
6.70 s for 2 and 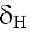 6.55s for 3. Table 2 shows that this part of the three molecules is almost the same, because they have very similar data for the carbons C-2 to C-10.
6.55s for 3. Table 2 shows that this part of the three molecules is almost the same, because they have very similar data for the carbons C-2 to C-10.
The structural difference of compounds 1, 2 and 3 is in the ring B, specifically at position C-3' (Figure 2). Thus, the position 3' is protonated in 2 showing its :H NMR spectrum one disubstituted aromatic system with two doublets orto-coupled, each one for 2 protons, at 7.94 d and 7.02 d ppm. Whereas 3 has an hydroxyl group at C-3' showing again the system meta, orto-meta, orto already observed in 1, but this time the compound has only three methoxy groups, previously assigned to the ring A, then the position C-3' has to have an OH group. This assignment is confirmed by the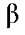 displacement of C-2' (4 ppm) in relation to compound 1. All assignments are showed in Table 2. In addition, the NMR data of compound 2 were compared with those reported in the literature [11] finding almost the same data but the carbons C-7, C-9 and C-5 were wrong assigned in the literature (interchanged) in agreement of the above discussion for the ring A. For the compound 3 the NMR:H signals were compared with bibliographic data for the same compound [15] but the NMR13C data were compared with bibliographic data finding for similar compounds [11], [14], [15] confirming the assignments done in this paper.
displacement of C-2' (4 ppm) in relation to compound 1. All assignments are showed in Table 2. In addition, the NMR data of compound 2 were compared with those reported in the literature [11] finding almost the same data but the carbons C-7, C-9 and C-5 were wrong assigned in the literature (interchanged) in agreement of the above discussion for the ring A. For the compound 3 the NMR:H signals were compared with bibliographic data for the same compound [15] but the NMR13C data were compared with bibliographic data finding for similar compounds [11], [14], [15] confirming the assignments done in this paper.
EXPERIMENTAL SECTION Plant material
Baccharis pentlandii leaves were collected in the city of La Paz, Bolivia (Cota Cota) at 3422 m.a.s.l., 16 ° 32,270 'S 68 ° 4016' W and was identified by Esther Valenzuela expert of National Herbarium of Bolivia where an specimen is stored.
Materials and methods
All of the solvents used were previously distilled. The spectra of 1D and 2D NMR were obtained on a Bruker NMR spectrometer of 300 MHz in the Chemical Research Institute (IIQ-UMSA) using CDCl3 as solvent. The UV-Vis spectra were obtained in the spectrophotometer Thermo Scientific GENESYS 10S from the same institute using MeOH as solvent. The chromatographic fractionation was performed using Silicagel 60G and Sephadex LH-20 columns. The chromatographic monitoring was performed using Thin Layer Chromatography (TLC) using 5% H2SO4 and FeCl3 in ethanol at 5% m/v as developers.
Extraction and Isolation
The leaves were cleaned, dried in the absence of solar radiation and ground. The extraction was carried out using EtOH 96% as solvent (100g per 1000 ml) the dry EtOH extract yield 8.8%. This extract was submitted to a liquid/liquid partition between methanol and petroleum ether forming two phases, the methanol phase was dried and extracted with a mixture of (CH2Cl2: MeOH 80:20). This medium polar extract (organic phase) was basified with KOH obtaining two phases: the aqueous was treated with HCl yielding a precipitate of a phenolic extract of medium polarity called EFPM [2]. EFPM performance relative to the ethanol extract is 53.9%.
Nine grams of EFPM were submitted to a VLC in Silicagel G60, using as eluents mixtures of petroleum ether and ethyl acetate in order of increasing polarity, obtaining the compound 1 with impurities and compound 2 pure in an amount of 45 mg. Finally the compound 1 was purified through a molecular exclusion chromatography Sephadex LH-20 isolating 29 mg of the pure metabolite.
An additional column of Silicagel was performed to obtain the compound 3 employing mixtures of dichloromethane and methanol in order of increasing polarity, obtaining 20 mg of pure compound.
Compound 1 (5,4'-dihydroxy-6,7,8,5'-tetramethoxyflavone; 8-Methoxycirsilineol) C19 H18 8O8 yellow crystals; Molecular Weight: 374.34 g / mol; UV in MeOH 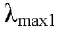 293 nm and
293 nm and 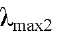 346 nm; 1H and 13C NMR (Tables 1 and 2). Compound 2 (5.4 '-dihydroxy-6 ,7,8-trimetoxiflavona Xantomicrol) yellow crystals of mp 227-229 ° C Molecular Formula C18H16O7, Molecular Weight 344.09g/mol, UV in MeOH
346 nm; 1H and 13C NMR (Tables 1 and 2). Compound 2 (5.4 '-dihydroxy-6 ,7,8-trimetoxiflavona Xantomicrol) yellow crystals of mp 227-229 ° C Molecular Formula C18H16O7, Molecular Weight 344.09g/mol, UV in MeOH 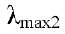 294 nm and
294 nm and 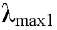 333nm; RMN1H and 13C (Table 2).
333nm; RMN1H and 13C (Table 2).
Compound 3 (5.4',5'-trihydroxy-6 ,7,8-trimetoxiflavona Sideritoflavone). Yellow crystals; Q8H16O8 Molecular Formula, Molecular Weight 360.315 g / mol, UV in MeOH 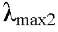 293 nm and
293 nm and 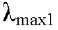 349 nm; RMN1H and 13C (Table 2)
349 nm; RMN1H and 13C (Table 2)
Notas
1 Corresponding author: giovyalmanza@yahoo.com.ar
REFERENCES
[1] ALMANZA G. R, & SALCEDO L. De la Planta al Medicamento. Investigaciones de Baccharis latifolia (Chilca) Primera Edición, UMSA, Noviembre de 2011, La Paz, Bolivia. [ Links ]
[2] ESCOBAR, Z., FLORES, Y., TEJEDA, L., ALVARADO, J. A., STERNER, O., AND. ALMANZA, G. R. Revista Boliviana de Química, 2009, 26 (2), 111. [ Links ]
[3] ABAD, M. J. & BERMEJO, P. ARKIVOC, 2007, 7, 76-96. [ Links ]
[4] GIULIANO, A. Darwiniana, 2001, 39, 131-154 [ Links ]
[5] GIRAULT L.Kallawayas, curanderos itinerantes de los Andes. Investigación sobre plantas medicinales y mágicas. Bolivia. Unicef, Quipus, 1987, La Paz -Bolivia [ Links ]
[6] VIDAURRE DE LA RIVA, P. Plantas medicinales en los Andes de Bolivia, Botanica Económica de los Andes Centrales, Ed. M. Moraes, B. 0llgaard, L. P. Kvist, F. Borchsenius & H. Balslev 2006, 268-284, La Paz-Bolivia [ Links ]
[7] FREIRE, E.S., URTUBEY, E. & GIULIANO, A. D. Caldasia, 2007, 29, 23-38. [ Links ]
[8] ABAD, M. J., BESSA, A.L., BALLARIN, B., ARAGÓN, O., GONZALES, E, BERMEJO, P. Journal of Ethnopharmacology, 2006, 103, 338-344 [ Links ]
[9] GONZALES, E., VILLCA, T., LOZA, R. Revista Boliviana de Química, 2007, 24 (1), 41- 44. [ Links ]
[10] FERESHTEH, J., SOLTAN A., NAHID, R. and MASSOUD, M. Phytochemistry, 2005, 66, 1581-1592 [ Links ]
[11] BRAHMI, Z., NIWA, H., YAMASATO, M., SHIGETO, S., KUSAKARI, Y., SUGAYA, K., ONOSE, J., & ABE, N. Bioscience, Biotechnology and Biochemistry, 2011, 75 (11), 2237-2239 [ Links ]
[12] SERGEEV, I. N., HO, C. T., LI, S., COLBY, J. AND DUSHENKOV, S. Molecular Nutrition & Food Research, 2007, 51, 1478 - 1484 [ Links ]
[13] SANCHEZ, I., CALDERÓN, J., RUIZ, B., TELLEZ, J., CALZADA, L. AND TABOADA, J. Phytotherapy Research, 2001, 15, 290-293. [ Links ]
[14] NGUYEN-HAI, N., YONG, K., YOUNG-JAE, Y., DONG-HO, H., HWAN-MOOK, K. & BYUNG-ZUN, A. Natural Product Letters, 2004, 18 (6), 485-491 [ Links ]
[15] BENKINIOUAR, R., TOUIL, A., ZAIDI, F., RHOUATI, S., CHOSSON, E., SEGUIN, E., COMTE, G., BELLVERT, F.Journal de la Société Algérienne de Chimie, 2010, 20 (1), 11-15 [ Links ]











 uBio
uBio