Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Boliviana de Química
On-line version ISSN 0250-5460
Rev. Bol. Quim vol.28 no.1 La Paz 2011
ARTICULO ORIGINAL
PHYSICOCHEMICAL AND INFRARED SPECTRAL PROPERTIES OF BIODIESEL FUELS SYNTHESIZED FROM SOME VEGETABLE OILS
Edgar A. Coronel C.
Instituto de Investigaciones Químicas – calles 27 y Andrés Bello Cota Cota, Campus Universidad Mayor de San Andrés, La Paz-Bolivia
Keywords: Biodiesel synthesis, Biodiesel-diesel mixtures, Biodiesel IR-FT spectra
ABSTRACT
A set of vegetable oils from Bolivian rain forests have been converted into biodiesel by base-catalyzed transesterification of fatty acids. Data regarding biodiesel properties dealing with feasibility of quality control procedures were obtained. Biodiesels have dynamic viscosity values in the range from 5.29 to 8.24 mPa.s at 20°C, and viscosity temperature dependences up to 90°C obey exponential functions. Dielectric constant values are between 3.75 and 4.34, whereas for diesel fuel is 2.35 ± 0.25. Measured diesel fuel density is 0.857 ± 0.015 g/cm³ at 20°C, while biodiesel densities are about 0.879 g/cm³. Temperature dependent specific gravity changes gave linear behavior between 20°C and 80°C. Heats of combustion values expressed in kJ/g are in the range 46.37 - 55.56 and a value of 49.42±2.20 for diesel fuel as reference. Measured differences do not depend on oils composition and could be attributed to the synthesis procedure. Neither water nor glycerol effects has been detected as meaningful impurities. Biodiesel-diesel fuel standard mixtures gave straight lines as calibration procedure to quantify biodiesel by infrared spectroscopy.
Corresponding author: edgar-coronel@daad-alumni.de
INTRODUCTION
Although Diesel engine was invented for using with fuels such as vegetable oils, petroleum diesel fuels have been used during last decades. However, mineral oils are nonrenewable resources and then companies and research groups are looking for new energy sources. Petroleum diesel has some environmental disadvantages when it is burned into a engine, sulfur and nitrogen contents contribute to the acid rain. On the other hand, biodiesel does not have those characteristics and can be obtained from renewable extensive farming of different seeds such as soybean, sunflower and others. A limitation of pure biodiesel fuels are their high viscosity at low temperatures. However, this trouble has been partially solved by using diesel fuel – biodiesel mixtures containing 2 or 5 % of biodiesel (1).
Biodiesel synthesis through transesterification of triglycerides using methanol under presence of a strong base, produce a mixture of long chain methyl esters and glycerol as by-product. Methanol acts in this case as transesterificant agent, however, ethanol can also be used.(2).
Biodiesel synthesis simplicity and common reagents availability, made possible a variety of experiments for teaching purposes, (3),(4). Detailed studies concerning analysis of fatty acids esters in biodiesel were also performed,(5). Until now, a variety of methods have been tested in order to produce biodiesel from different raw materials (6),(7) , experimental conditions of reactions (8 ), (9) , using different catalysts, (10),(11),(12). However, few studies concerning electrical properties of biofuels have been developed, (13). Research trends from different points of view including health topics are published currently, (14),(15),(16). A kind of biodiesel obtained by esterification process from waste materials such as animal fats without affecting the food chain has been also reported, (17). Analytical work faced with counterfeited biodiesel-diesel fuel mixtures using spectrofluorimetry has been published,(18). A concerned review about biofuels appeared recently,(19).
The aim of this work is to show how a variety of biodiesel fuels can be synthesized through two ways from Bolivian vegetable oils. Biodiesel properties such as viscosity, density, dielectric constant and heat of combustion data are reported. Because of thermal effect on biodiesel during its usage, viscosity and density temperature dependent data are also presented. In order to develop a quantitative analytical method for biodiesel contents in diesel fuel-biodiesel mixtures, FT-Infrared spectroscopy has been applied. Biodiesel properties as experimental data, are compared with measured properties made on petroleum diesel fuel available commercially.
RESULTS, DISCUSSION
The most representative results of measured and calculated biodiesel properties are shown in the tables 1 and 2. Figures 1 to 3, show data obtained according to the experimental section. Figure 4, shows typical IR-FT calibration straight lines for analytical purposes.
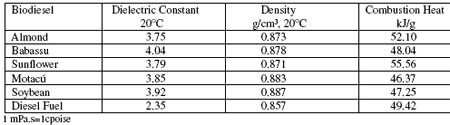
Table 1. Averaged experimental values of physicochemical properties of synthesized biodiesel fuels obtained from vegetable oils.
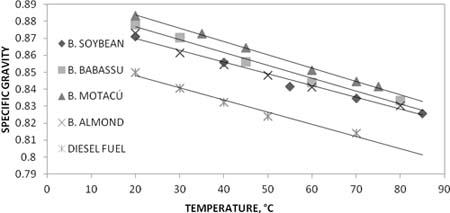
Fig.1. Specific gravities as a function of temperature for synthesized biodiesel and diesel fuel.
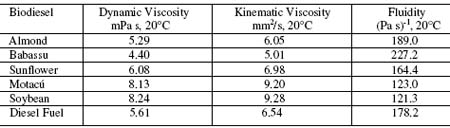
Table 2. Measured and calculated properties of synthesized biodiesel fuels.
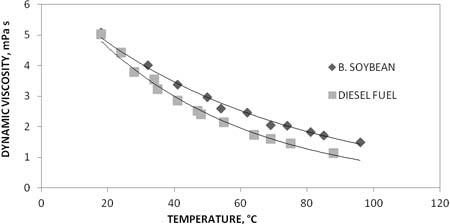
Fig. 2. Comparison of temperature-dependent of viscosity changes for soybeanbiodiesel and petroleum diesel.
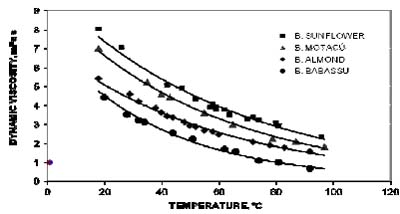
Fig.3. Temperature-dependent viscosity changes for others synthesized biodiesel.
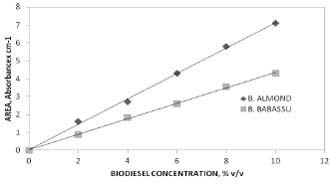
Fig.4. Some calibration straight lines showing infrared spectra results to quantify biodiesel in diesel fuel-biodiesel mixtures.
EXPERIMENTAL
Procedure A. All reactions were carried out using a cooled reflux Friedrich type condenser attached to a 250 mL three-necked round-bottomed flask equipped with a magnetic stir bar, a mercury thermometer and a glass stopper. Reagent grade (Merck) ethanol and sodium hydroxide were used without further purification , 25 mL of methanol and 0.870 g of sodium hydroxide as pellets were necessary in all cases. Vegetable oils as starting materials were purchased from commercial suppliers, and they have not modified additionally. A typical volume of vegetable oil to be transformed was 87 mL. These substances were mixed together into the reaction flask and under constant stirring, the mixture was gently heated from room temperature to 55°C. The reaction temperature was kept constant and allowing to proceed during one hour. After that, the raw product was cooled to room temperature. A clear-brown heavy liquid layer or glycerol was removed from the bottom using a pipette. Biodiesel is less dense than glycerol and has a yellow-brown color. This fraction was stirred and heated for additional 30 minutes. The rest of glycerol was removed using a separation funnel. The biodiesel layer was repeatedly washed with fractions of distilled water (20 mL) until neutrality of wash water. Washed biodiesel liquids are more yellow than brown and form emulsions which turn clear after standing up for 4 or 5 days.
Procedure B. Using similar quantities of substances, sodium hydroxide was mixed with methanol, stirring and heating the mixture slowly up to 55°C during 15 minutes until pellets dissolution. Sodium metoxide is highly corrosive, then it has been carefully handled. A volume of 87 mL of vegetable oil was heated near 55°C separately, and then mixed with the metoxide stirring continuously for one hour. When a reaction is over, the mixture was cooled and the upper part layer or biodiesel was separated. The measured biodiesel yields based on volumes were near 100%. Glycerol solidifies at room temperature. Biodiesel was rinsed out with water and a white cloudy emulsion appeared.
The following vegetable oils were used as reactants, motacú palm oil (Scheelea princeps), almond oil (Prunus dulcis), babassu palm oil (Orbignya phalerata), sunflower oil (Helianthus annuus) and soybean oil (Glycine max). All of these oils are from trees or plants that grow in Bolivian rain forests.
Viscosity. A Canon-Fenske glass capillary viscometer tube number 75 has been calibrated with distilled water at 20°C. Each time about 10 mL of different sample has been tested. Samples were measured in the range between 20°C and 100°C, by immersing into a thermostated oil bath E.H. SARGENT provided with a temperature controller. Additional calibrations up to 80°C at intervals of ten degrees were also performed. At equilibrium, sample temperature was measured with a K type thermocouple and its digital reading output. A digital chronometer was used for measuring the time required to flow between marked lines on viscometer tube.
Heat of combustion. Samples of 0.5 ml each, were burned up into a PARR model 1341 bomb calorimeter containing oxygen at 20 atmospheres. The calorimeter has been calibrated according to standard protocol recommended for it. Calibration pellets of benzoic acid of 1.035 g gave a calorimeter constant of 2425 cal/ °C in average. Burned mass sample fuels were determined from density data.
Dielectric constant. A chemical oscillometer, SARGENT model V operating at 5 MHz of electric field, provided with a glass tube cell of 12 mm internal diameter was used. The cell to contain 2.5 mL of liquid, was wrapped and glued with two external thin aluminum foils arranged coaxially. A cell holder and oscillometer have been connected by a coaxial cable of 20 cm long with Amphenol PL-259 plugs. Two procedures of calibration were applied , one using two organic solvent as standards according to the protocol for it or using five different pure solvents and their well established dielectric constant values in order to obtain a calibration straight line from readings. In the last case, n-hexane, bencene, carbon tetrachloride, 1,4 dioxane and chloroform, this set covers the range from 1.80 to 4.80 units of dielectric constants at 20°C.
Density and specific gravity. Density determinations were carried out using a Gay-Lussac bottle with perforated glass stopper and calibrated with water at 20°C. Averaged bottle mass and volumes were 8.2299 g and 1.7945 mL respectively. Specific gravities as a function of temperature were measured with a re-calibrated hydrometer for testing petroleum oils in the range 0.700-1.000 together with a 50 mL glass cylinder to contain a liquid sample. A water circulating bath for warming up was useful. When measuring samples, we used a longer thermal equilibration time. As reference liquid fuel, petroleum diesel samples have been measured.
Infrared quantitative analysis of diesel-biodiesel mixtures. Infrared spectra were recorded in the range 4000 – 400 cm -1 on a Perkin Elmer Spectrum BX, Fourier Transform spectrometer. A horizontal attenuated total reflection accessory (HATR) provided with a zinc selenide crystal flat sample holder was used. Either pure or in solution, biodiesel infrared spectra show a well defined strong peak due to carbonyl group at 1745 cm-1 or 1737 cm -1 .In absorbance mode, an appropriate base line under peak were determined. Corrected areas have been obtained for a set of standards using petroleum diesel as solvent. The following biodiesel volumes were diluted to 5 mL: 0.1, 0.2, 0.3, 0.4 and 0.5mL, giving an analytical range of concentration up to 10 % v/v. Approximately, 0.5 mL of sample was enough to record a spectrum.
CONCLUSIONS
The most amenable way to obtain a series of biodiesel is the procedure B, because two separated stages are involved. Each reaction stage not only can be followed under controlled conditions, but also the conversions may be looked into. Biodiesel densities at 20°C, do not vary substantially, about 1% deviation for the set. However, in the range from 20°C to 80°C there is a common behavior in terms of linearity and the same deviation among them. Figures 2 and 3, show temperature dependent changes of viscosity for different biodiesel samples. between 20°C and 100°C. In these cases, a kind of Arrhenius equation may be applicable. However, since the scrambled composition of fatty acid methyl esters the term of activation energy cannot be explained evenly. An observation common to all synthesized biodiesel is that dielectric constant, density, heat of combustion and viscosity are somewhat high than the measured properties of petroleum diesel used as reference. Taken Dielectric constant data trend to be almost twice the reference; nevertheless, it does not mean an abnormal characteristic because data are still in the low dielectric constant range compared with other liquid fuels.
Petroleum diesel has a dynamic viscosity value of 5.61 mPa s at 20° C , the other ones are in the range from 4.40 to 8.24 mPa s. These differences could be attributed to preparation procedures in which reaction times along with rinsing steps are involved. Kinematic viscosity and fluidity values were calculated from technical definitions, then are presented just for comparison purposes. There is no correlation between major fatty acid contents of vegetable oils and measured properties. Among sunflower and soybean oils, they have 55% of linoleic acid and 19% of oleic acid in average. On the other hand, babassu palm oil with a content of 45% of lauric has similar measured properties data set. Different biodiesel viscosity values at 20°C cannot be correlated with fatty acid contents in the other cases such as almond oil with 68% of oleic acid and 25% of linoleic acid or motacú oil with 45% of palmitic acid and 40% of oleic acid. As a result, data given above do not permit us to distinguish between biodiesels arising from different starting vegetable oils. Despite the viscosity dependences with temperature obey exponential behavior, differences could be caused by impurities such as glycerol because it has higher viscosity at 20°C (1.408 Pa s). However, it seems less probable due to the measured densities and viscosities at 20°C are almost common for all biodiesels. An additional criteria faced with biodiesel purity, are their dielectric constants that did not show wide variations. If there were an important effect of glycerol as contaminant, their dielectric constants would increase due to glycerol dielectric constant is 42.5 at 25°C. Moreover, a small quantity of water content in biodiesel can also increase dielectric constants because water has a very high dielectric constant, 80.10 at 20°C. Studied properties of an isolated biodiesel will be overwhelmed by petroleum diesel properties in case of mixed fuels. According to the figure 4, infrared absorbance data for biodiesel-diesel fuel standards, areas under carbonyl peaks centered at 1745 cm-1 or 1737 cm-1 showed to be useful to plot calibration straight lines as absorbance x cmˉ¹ vs. biodiesel concentrations up to 10% v/v or B10 fuel. The analytical range may be extended to 20% or B20 fuel. In addition, due to a infrared spectrometer records the whole spectrum, counterfeited biodiesel-diesel fuels can be detected simultaneously. Finally, most of the data reported here are agree with DIN (Germany) and ASTM (U.S.A.) standard values for FAME (fatty acid methyl ester).
ACKNOWLEDGEMENTS
The author thanks the Instituto de Investigaciones Químicas (I.I.Q.), Facultad de Ciencias Puras y Naturales, Universidad Mayor de San Andrés, Franco-Boliviano High School of La Paz for providing us some reagents and Ms. Catalina Ramirez for encouraging and assistance.
REFERENCES
(1) Oliveira J.R., et al.,Quím. Nova,2007, 30, 600 [ Links ]
(2) Clarke N.R. et al., J. Chem. Educ.,2006, 83, 257 [ Links ]
(3) Bucholtz E.C., J. Chem. Educ.,2007, 84, 296 [ Links ]
(4) Guarieiro L.L.N., Pinto A.C.,Fernandes P., Moura N., Quim. Nova, 2008,31,421 [ Links ]
(5) Urioste D, Castro M.B.A. Biaggio F.C. and Castro H.F. Quím.Nova, 2008, 31,407 [ Links ]
(6) S.S. Varun Shankar, et al., Intl. J. of Chem Eng. and Appl., 2010,1, No.1. [ Links ]
(7) A.V. Kurzin,A.N. Evdokimov, O.S. Pavlova and V.B. Antipine, Russian Journal of Applied Chemistry, 80, 842 [ Links ]
(8) WSEAS Transactions on Environment and Development, 2008, 4, 4. [ Links ]
(9) R. Yilong, H. Adam, Z. Rabitah, Journal of Biobased Materials and Bioenergy, 2010,4, 7986. [ Links ]
(10) A.J. Gotch, A.J. Reeder, and A. McCormick, Journal of Undergraduate Chemistry Research, 2008, 4, 58 [ Links ]
(11) F. Yan, Z. Yuan, et al., J. of Fuel Chemistry and Technology, 2010, 38, 281 [ Links ]
(12) Leilei Xu, et al., J. Mater. Chem. 2009, 19, 8571 [ Links ]
(13) P.A. Sorichetti, S.D. Romano, Physics and Chemistry of Liquids, 2005, 43, 37 [ Links ]
(14) Takashi Hoshino,Yusaku Iwata, Hiroshi Koseki, Thermal Science, 2007, 11, 87 [ Links ]
(15) K. Kohse-Hoinghaus, et al., Angewandte Chemie, Intl. Edition, 2010, 49, 3572 [ Links ]
(16) J.M. de Brito, L. Belotti, et al., Toxicol. Sci , 2010, 116, 67 [ Links ]
(17) C.I.Bianchi,D.C.Boffito,C. Pirola,V.Ragaini, Catal. Lett.,2010, 134, 179 [ Links ]
(18) M. Meira, et al.,Quim.Nova, 2011, 34, 621 [ Links ]
(19) D. Biello, Scientific American, 2011, 305, 2 [ Links ]