Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Boliviana de Química
On-line version ISSN 0250-5460
Rev. Bol. Quim vol.28 no.1 La Paz 2011
ARTICULO ORIGINAL
STUDIES ON COMPLEXATION IN SOLUTION WITH PAPER IONOPHORETIC TECHNIQUE [THE SYSTEM MERCURY(II) / NICKEL(II) / LEAD(II) – HYDROXYPROLINE]
Brij B. Tewari
Department of Chemistry, Faculty of Natural Sciences, University of Guyana, P. O. Box: 101110, Georgetown, Guyana
Keywords: Ionophoretic technique, mercury(II) complexes, nickel(II) complexes, lead(II) complexes, hydroxyproline, overall mobility , stability constant.
ABSTRACT
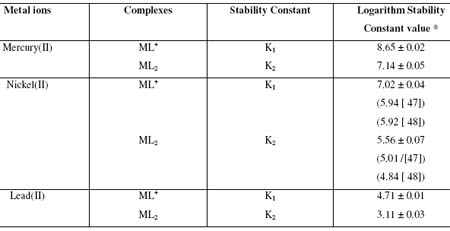
A new method, paper electrophoresis, involving the use of an ionophoretic technique is described for the study of equilibria in solution. This method is based on migration of a metal ion spot (M), under an electric field at various pHs of background electrolyte. A plot of pHs versus mobility of metal ion spots provide information about the formation of metal complexes allowing calculation of the stability of these complexes. The stability constant of ML and ML2 complex species of Hg(II), Ni(II) and Pb(II) with hydroxyproline were determined at an ionic strength of 0.1 M (perchloric acid) and at a temperature of 35º C. The stability constants of ML and ML2 complexes of metal(II) – hydroxyproline have been found to be (8.65 ± 0.02; 7.14 ± 0.05), (7.02 ± 0.04; 5.56 ± 0.07) and (4.71 ± 0.01; 3.11 ± 0.03) (log k values) for Hg2+, Ni2+ and Pb2+ complexes, respectively.
Corresponding author: brijtew@yahoo.com
INTRODUCTION
The importance of metal ions to vital functions in living systems and for the well being of living organism is now well-established [1]. Nickel classified as beneficial and mercury as well as lead as toxic metals, respectively in biological systems. Average nickel content of healthy human body weight (70 kg) is 0.01g. The recommended elements in human diet (mg / day) are (0.30 – 0.50), (0.06 – 0.50) and (0.004 – 0.020) for nickel, lead and mercury, respectively. Nickel is integral component of the enzyme ureases: may be involved into action of hydrogenases. Mercury is harmful even a concentration of 0.03 ppm in drinking water. Mercury deactivates sulfur containing enzymes with active – SH groups, affects brain cells and the central nervous system. Lead destroys sulfur containing proteins and enzymes, causes damage in DNA, RNA brain and Central nervous system functions. Lead also inhibits several steps in the formation of hemoglobin mercury nickel and lead have several significant applications in biological systems [2-23]. Hydroxyproline is a non-essential amino acid, which means that it is manufactured from other amino acids, which means that it is manufactured from other amino acids in the liver and it does not have to be obtained directly through the diet. Hydroxyproline is necessary for the construction of the bodys major structural protein, collagen. Defects in collagen synthesis lead to easy bruising, internal bleeding, breakdown of connective tissue of the ligaments and tendons and increased risk to blood vessel damage. Hydroxyproline has several significant applications in biological systems [24-33]. Publications [34-38] from our laboratory described a new method for the study of metal complexes by paper electrophoretic technique. A search of literature indicated that few reports available on binary complexes of nickel(II) with hydroxyproline, but no report available on binary complexes of nickel(II) with hydroxyproline, but no report available on binary complexes of mercury(II) and lead(II) with hydroxyproline. In view of this, attempts were made to establish the optimum conditions for metal(II) – hydroxyproline complex formation. In addition, present work describe a paper electrophoretic method for the determination of stability constants of these complexes.
RESULTS
Chemical literature [39, 40] confirms that anionic species of amino acids are the sole ligating species of metal ions. The plot of overall electrophoretic mobility of metal spot against pH gives a curve with a number of plateaus shown in Figure 4. A plateau is obviously an indication of a pH range where speed is practically constant. This is possible only when a particular complex is overwhelmingly formed. Thus every plateau indicates the formation of certain complex species. The first plateau corresponds to a region in which metal ions are uncomplexed. In this low pH region protonated species of hydroxyproline is non-complexing. Beyond this region, metal ion spots have progressively decreasing velocity, and hence complexation of metal ions should be taking place with some anionic species of ligand hydroxyproline whose concentration increases progressively with increase of pH.
Figure 4 shows that second plateau in each case with positive mobility indicating the formation of 1:1 complex of cationic nature. The mobility register a downward trend ultimately resulting in a third plateau obviously corresponds to overwhelmingly formation of 1:2 complex of neutral nature. It is significant that these studies give clear evidence of the complexation of anionic species of hydroxyproline with Hg2+, Ni2+ and Pb2+ metal ions forming two varieties of binary complexes of 1:1 and 1:2 composition. The prominent ligating properties of the unprotonated anionic species of hydroxyproline ruling out any such property of zwitterions [41, 42]. In view of the above observation, the complexation of metal ion with hydroxyproline anion may be represented as:
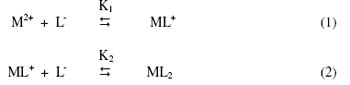
where M2+ is Hg2+ Ni2+ and Pb2+ metal ions; [L-] is the hydroxyproline anion; K1 and K2 are the first and second stability constants, respectively. The metal spot on the paper is thus a combination of uncomplexed metal ions; 1:1 and 1:2 metal complexes. The spot is moving under the influence of electric field and the overall mobility U is given by equation [43].
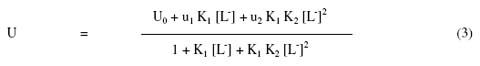
where u0, u1 and u2 are mobilities of uncomplexed metal ion, 1:1 metal complex and 1:2 metal complex, respectively. Equation (3) has been used for calculating stability constants of the complex of metal ions with hydroxyproline. For the calculation of the first stability constant K1 the region between the first and second plateau is pertinent. The overall mobility U will be equal to the arithmetic mean of mobility of uncomplexed metal ion, u0, and that of first complex u1 at a pH where K1 = 1/ [L-]. With the help of dissociation constants of hydroxyproline [electrophoretically obtained value, k1 = 101.80; k2 = 109.46] the concentration of the hydroxyproline anion [L-] is determined for the pH, from which K1 can be calculated. The mode of dissociation of pure hydroxyproline can be represented as:
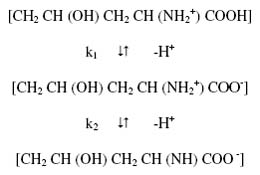
The concentration of complexing hydroxyproline [L-] is calculated with the help of equation
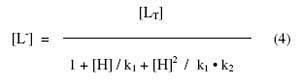
where [LT] is total concentration of hydroxyproline (0.01 M); k1 and k2 are first and second dissociation constant of pure hydroxyproline respectively. The stability constant, K2, of second complex can be calculated by taking into consideration the region between second and third plateau of mobility curve. The calculated values of K1 and K2 are given in Table 1.
Table 1. Stability constants of binary complexes of mercury(II), nickel(II) and lead(II) with hydroxyproline.
DISCUSSION
Stability constants of metal ion complexes with hydroxyproline follow the order:
mercury(II) > nickel(II) > lead(II)
The high stability constant values of mercury(II) – hydroxyproline complexes indicate strong bonding between mercury(II) cation and hydroxyproline anion. While low stability constant value between lead(II) - hydroxyproline complexes indicate weak bonding between lead(II) cation and hydroxyproline anion. The high stability of mercury(II) – hydroxyproline complexes may be ascribed to be its greater affinity for the oxygen donor ligands. It is also observed from Table 1 that first and second stability constants values of ML+ and ML2 complexes follow the order : log K1 > log K2 ,in each system. It is therefore inferred that coordinating tendency of a ligand decreases with the higher state of aggregation [44]. The molecular structure of hydroxyproline is as follows:
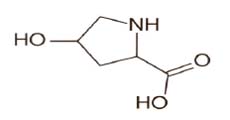
It is also observed from Table 1 that the stability constant values are approximately similar to literature values. The slight deviation in the values obtained from different sources is mainly due to the difference in temperature, ionic strength, and experimental conditions used by different workers. The stability constants of metal complexes, can be very easily calculated by this technique; therefore the present method is advantageous over other methods (viz. polarography, potentiometry, solubility, etc.) reported in chemical literature for the determination of stability constants of metal complexes. The present technique is limited to charged species, and the precision of the method is not high as other physicochemical methods. However, uncertainty in the results is ± 5 %. It is not felt that it can replace the most reliable methods, although it is new approach worth developing. The proposed structure of the ML2 complex is given as follows:
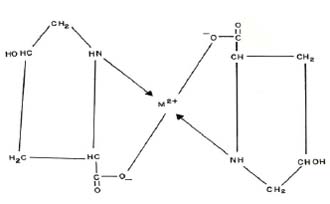
CONCLUDING REMARKS
It may be concluded from present study that mercury(II), nickel(II) and lead(II) are significant in biological systems but as such they are toxic, the hydroxyproline may be used to reduce the level of these metal ions in the biological system. Mercury(II) – hydroxyproline and lead(II) – hydroxyproline complexes are found to have higher and lower stability constant values, respectively. The ML2 complexlees are found to have low stability constant values and less stable in comparison to ML complexes in each system. Present advanced electrophoretic technique has thus proved to be helpful in deciding whether a complex system is formed or not, and if it is formed its stability constant can also be determined. Biologically significant mercury(II), nickel(II) and lead(II) complexes with hydroxyproline can be prepared on large at particular pH of the background electrolyte solutions.
EXPERIMENTAL
Instruments
A Systronics (Naroda, India) paper electrophoresis equipment horizontal-cum-vertical type, model 604, has been used. The apparatus consisted of a PVC moulded double tank vessel. In our laboratory, significant change to the instrument has been made. Two hollow rectangular plates covered with thin polythene sheets have been used through which thermostated water is run for controlling the temperature. The tanks were closed with a transparent PVC moulded lid. The whole assembly is tight, which prevents moisture changes, which may upset the equilibria in a paper strip. This assembly design thus keeps to a minimum the disturbing effects of evaporation from the unwanted liquid flow in the paper. Each electrolyte tank contains a separate electrode chamber. The auxiliary unit is specially designed to operate either in voltage mode or in current mode.
An Elico (Hyderabad, India), Model L1-10, having glass and calomel electrodes assembly working on 220 V/50 Hz established a.c. mains, was employed for pH measurements. The electrophoresis cell showing sandwiched paper strips and water supply are shown in Figure 1.
Chemicals
Mercury(II), nickel(II) and lead(II) perchlorate solutions were prepared by preliminary precipitation of metal carbonates from a 0.1 M solution of sodium carbonate (AnalaR grade, BDH, Poole, UK). The precipitates were thoroughly washed with boiling water and treated with calculated amounts of 1 % perchloric acid. The resulting mixture was heated to boiling on a water bath and then filtered. The metal content of the filtrates were determined and final concentration was kept at 0.005 M [45, 46]. The position of the Ni2+ spots on the paper at the end of the experiment was detected using ammonical dimethylglyoxime (DMG), that of Pb2+ detected by 0.1% solution of 1-(2-pyridylazo) – 2- naphthol (PAN) (Merck, Darmstadt, Germany) in ethanol, that of Hg2+ detected using hydrogen sulphide in water. The 0.005 M glucose (BDH, AnalaR) solution was prepared in water and used as an indicator for the correction due to electroosmosis. A saturated aqueous solution (0.9 mL) of silver nitrate was diluted with acetone to 20 mL. Glucose was detected by spraying with this silver nitrate solution and then with 2% ethanolic solution of sodium hydroxide, when a black spot was formed. Paper strips showing the positions of the metal ion spots after electrophoresis are shown in Figure 2.
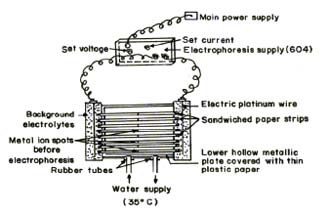
Fig.1 Electrophoresis cell showing sandwiched paper strips
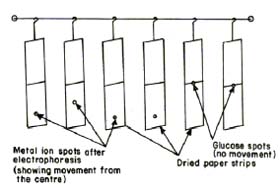
Fig.2 Paper strips showing position of metal spots after electrophoresis
Background electrolyte (BGE)
Stock solution of 5.0 M perchloric acid was prepared from its 70% solution (SDS, AnalaR grade). 2.0 M sodium hydroxide and 0.5 M hydroxyproline (BDH, Poole, UK) solutions were prepared. The background electrolyte used in the study of binary complexes were 0.1 M perchloric acid and 0.1 M hydroxyproline. The system was maintained at various pH by the addition of sodium hydroxide.
Procedure
Whatman No. 1 filter paper for chromatography was used for the purpose of electrophoresis. For recording observation of particular metal ion, two strips were spotted with the metal ion solution along with additional two spotted with glucose using 1.0 ml pipette and then mounted on the insulated plate. Each of the two electrolyte vessels were filled with 150 ml of background electrolyte containing 0.1 M perchloric acid and 0.01 M hydroxyproline. The paper become moistened with the background electrolyte solutions due to diffusion. The second insulated plate was placed on paper strips and then thermostated water (35° C) was circulated in the plates to keep the temperature constant. The lid was then placed on the instrument to make it air tight. It was left for 10 minutes to insure wetting of strips. Subsequently a direct 200 Volts potential was applied between the electrodes. Electrophoresis was carried out for 60 minutes after which these strips were removed from the tank and dried. The metal ion and glucose spots were detected by specific reagents. The leading and tailing edge were measured from the marked centre point and the mean were taken. The distance moved by glucose was subtracted (in case of migration toward anode) to obtain correct path length. Migration towards anode and cathode were designated by negative and positive signs respectively. Electrophoretic observations of metal ions were recorded at various pH values of the background electrolyte obtained by adding NaOH solution, the ionic strength being maintained at 0.1 M. The observed mobility of migrant was calculated by using the formula.

where U = mobility of metal ion / complex ion; d = mean of duplicate distance travelled by metal ion / complex ion; dG = mean of duplicate distance travelled by glucose spot; x = field strength; t = time for electrophoresis. The scheme for paper electrophoresis set-up is shown in Figure 3.
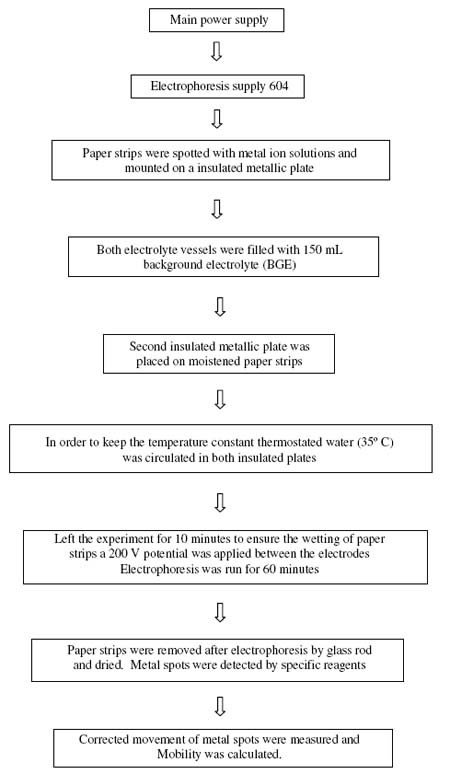
Fig. 3 The scheme for paper electrophoresis set-up
The protonation constants of pure hydroxyproline were determined by same paper electrophoretic technique. The two paper strips were spotted with pure hydroxyproline along with two other spotted with glucose using 0.1 M perchloric acid only in a background electrolyte. The electrophoresis was carried out for 60 minutes as for metal ions. The electrophoretic speed was calculated. The speeds of the metal ion / amino acid are reported with pH values. The individual speeds of the duplicate spots were found to be fairly equal. A plot of mobility against pH is shown in Figure 4.
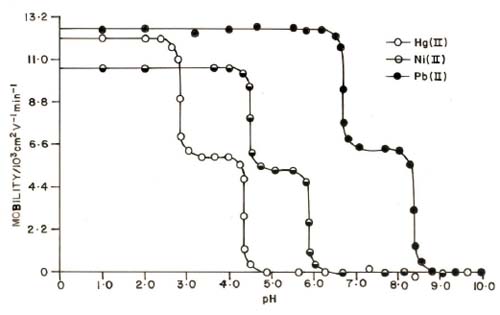
Figure 4. Mobility curves for the metal(II) – hydroxyproline. ![]() = Hg(II) – hydroxyproline = nickel(II) – hydroxyproline
= Hg(II) – hydroxyproline = nickel(II) – hydroxyproline ![]()
![]() = Pb(II) – hydroxyproline. pHs were maintained by addition of sodium hydroxide. Ionic strength = 0.1 M; temperature = 35° C. The paper strips were spotted with 0.1 ml of sample solution and glucose (for making osmotic corrections).
= Pb(II) – hydroxyproline. pHs were maintained by addition of sodium hydroxide. Ionic strength = 0.1 M; temperature = 35° C. The paper strips were spotted with 0.1 ml of sample solution and glucose (for making osmotic corrections).
REFERENCES
1. BANERJEA D., Everymans Sci. 1995, 6, 176. [ Links ]
2. KHAN M. A. K., WANG F., Environ. Toxicol. Chem. 2009, 28(8), 1567. [ Links ]
3. SHOKROLLAHI A., GHALDI M., SHAMSIPUR M., Quim. Nova. 2009, 32(1), 153. [ Links ]
4. XIE L., FLIPPIN J. L., DEIGHTON N., FUNK D. H., BUCHWALTERD. B., Environ. Sci. Technol. 1009, 43, 934.
5. LEE M. H., LEE S. W., KIM S. H., KANG C., KIM J. S., Org. Lett. 2009, 11(10), 2101. [ Links ]
6. SOUSA G. D., ZUCCHI T. D., ZUCCHI F. D., MILLER R. G., ANJOS R. M. A., POLI P., ZUCCHI T. M. A. D., Genet. Mol. Res. 2009, 8(2), 404. [ Links ]
7. JANA A., KIM J. S., JUNG H. S., BHARADWAJ P. K., Chem. Commun. 2009, P. 4417. [ Links ]
8. HUANG C. C., CHIEN M. –F., LIN K. –H., Interdisciplinary Studies on Environmental Chemistry – Biological Response to Contaminants, (eds. N. Hamamura, S. Suzuki, S. Mendo, C. M. Barroso, H. Iwata, S. Tanabe) Terrapub., 2010, p. 23. [ Links ]
9. KARTHIKEYAN J., PARAMESHWARA P., SHETTY A. N., Indian J. Chem. Technol. 2008, 15, 493. [ Links ]
10. SUMI D., J. Health Sci. 2008, 54(2), 267. [ Links ]
11. PRAKASH A. , GANGWAR M. P., SINGH K. K., J. Dev. Biol. Tissue Eng. 2011, 3(2), 13. [ Links ]
12. MOUNKIA K., ANUPAMA B., PRAGATHI J., GYANKUMASI C., J. Sci. Res. 2010, 2(3), 513. [ Links ]
13. SAEED S., RASHID N., ALI M., HUSSAIN R. , Eur. J. Chem. 2010, 1(3), 200. [ Links ]
14. CHANG E. L., SIMMERS C., KNIGHT D. A., Pharmaceuticals, 2010, 3, 1711. [ Links ]
15. GRUBEL K., FULLER A. L., CHAMBERS B. M., ARIF A. M. A. , BERREAU L. M. , Inorg. Chem. 2010, 49(3), 1071. [ Links ]
16. MANSOOR S. S., Int. J. Chem. Tech. Res., 2010, 2(1), 640. [ Links ]
17. NAALIYA J., Bayero J. Pure Appl. Sci. 2010, 3(1), 241. [ Links ]
18. KHAN M. R., KHAN M. M., Aust. J. Basic Appl. Sci. 2010, 4(6), 1036. [ Links ]
19. SIMMERS S. O., FAN C.-Y., YEOMAN K., WAKEFIELD J., RAMABHADRAN R., Curr. Chem. Genom.2011, 5, 1. [ Links ]
20. SILVEIRA E. A., LIZARDO J. H. F., SOUZA L. P. , STEFANON I. , VASSALLO D. V. , Braz. J. Med. Res. 2010, 43(5), 492. [ Links ]
21. OU-YANG H. D., MING -TZO W., Annu. Rev. Phys. Chem. 2010, 61, 421. [ Links ]
22. DING W. –X., World J. Biol. Chem. 2010, 1(1), 3. [ Links ]
23. IMANPOOR M. R., BAGHERI T., World J. Zoology 2010, 5(4), 278. [ Links ]
24. GULER G. , TURKOZER Z., OZGUR E., TOMRUK A., SEYHAN N.,KARASU C., Gen. Physiol. Biophys. 2009, 28, 47. [ Links ]
25. WEIS M. A., HUDSON D. M., KIM L., SCOTT M. , WU J. J. , EYRE D. R., J. Biol. Chem. 2010, 285(4), 2580. [ Links ]
26. ESTEVEZ J. M., KIELISZEWSKI M. J., KHITROV N., SOMERVILLE C., Plant Physiol. 2006, 142, 458. [ Links ]
27. HAN X., SHEN T., LOU H., Int. J. Mol. Sci. 2007, 8, 950. [ Links ]
28. XU C. , TAKAC T., BURBACH C., MENZEL D., SAMAJ J., BMC Plant Biol. 2011, 11, 38. [ Links ]
29. MUELLER C., EMMRICH J., JASTER R. , BRAUN D., LIEBE S. , SPARMANN G., World J. Gastroenterol. 2006, 12(10), 1569. [ Links ]
30. KAPTON D.M., PENNISTON K. L., DARRIET C., CRENSHAW T. D., NAKADA S. Y., J. Endourol. 2010, 24(3), 355. [ Links ]
31. TOYOOKA T., Curr. Pharma. Analysis, 2005, 1, 57. [ Links ]
32. LUKIVSHAYA O., PATSENKER E., LIS R., BUKO V. U., Eur. J. Clin.Invest. 2008, 38(5), 317. [ Links ]
33. INAN A., SURGIT O., SEN M., AKPINAR A., BOZER M., Turk J. Med. Sci. 2009, 39(5), 713. [ Links ]
34. TEWARI B. B., Trans. SAEST, 1995, 30(2), 76. [ Links ]
35. TEWARI B. B., Bull. Korean Chem. Soc. 2002, 23(5), 705. [ Links ]
36. TEWARI B. B., J. Electrochem. Soc. India 1994, 43, 111. [ Links ]
37. TEWARI B. B., J. Chem. Eng. Data 2010, 55(5), 1779 . [ Links ]
38. TEWARI B. B., Russ. J. Inorg. Chem. 2005, 50(1), 39. [ Links ]
39. LEVASON W., MCAULIFFE A. C., JOHUS M. D., Inorg. Nucl. Chem. Lett. 1977, 13, 123. [ Links ]
40. MUSHER K. W., NEAGLEY H. C., Inorg. Chem. 1975, 14, 1728. [ Links ]
41. BLACKBURN J. R., JONES M. M., J. Inorg. Nucl. Chem. 1973, 35, 1605. [ Links ]
42. SOKOL L. S., LAUSSEGGER H., ZOMPA L. J. , BRUBAKER C. S., J. Inorg. Nucl. Chem. 1971, 33, 3581. [ Links ]
43. JOKL V., J. Chromatogr., 1964, 6, 432. [ Links ]
44. JOSHI J. D., BHATTACHARYA P. K., J. Indian Chem. Soc. 1973, 50, 344. [ Links ]
45. KOLTHOFF I. M., BELCHER R., Volumetric Analysis, Intersciences Publisher Inc. New York, Vol. 3, 1957. [ Links ]
46. VOGEL A. I., Text Book of Quantitative Inorganic Analysis: Including Elementary Instrumental Analysis, 4th edition, Longman, London. 1978. [ Links ]
47. MARTELL A. E. , SMITH R. M., Critical Stability Constants, Vol. 1, Amino Acids, Plenum Press, New York, USA, 1974, p. 73. [ Links ]
SILLEN L. G. , MARTELL A. E., Stability Constants of Metal Ion Complexes, Special Publications No. 17, Chemical Society London, 1974, p. 457.