Revista Boliviana de Química
versión On-line ISSN 0250-5460
Rev. Bol. Quim v.27 n.1 La Paz ago. 2010
ARTICULO ORIGINAL
INTERACTION OF (-AMINOBUTENOIC ACID WITH DIVALENT BERYLLIUM(II) AND COBALT(II) IN CHEMICAL AND BIOLOGICAL SYSTEMS
Brij B. Tewari
Department of Chemistry, Faculty of Natural Sciences, University of Guyana, P. O. Box: 101110, Georgetown, Guyana
Keywords: Paper ionophoretic technique; overall mobility; beryllium(II) complexes; cobalt(II) complexes; aminobutenoic acid, stability constants.
ABSTRACT
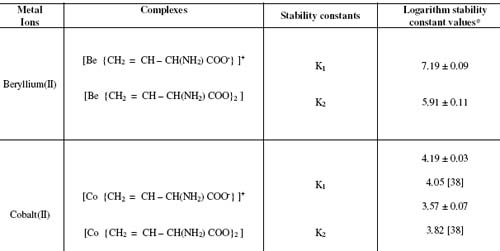
Paper electrophoresis has been applied to the study of metal complexes in solution and attempts have been made to determine the stability constants of complex species. The proportion of ionic species of (-aminobutenoic acid was varied by changing the pH of background electrolyte. The stability constants of ML and ML2 complexes of Be(II) - (-aminobutenoic and Co(II) - (-aminobutenoic acid complexes were found to be (7.19 ± 0.09, 5.91 ± 0.11) and (4.19 ± 0.03, 3.57 ± 0.07) (logarithm stability constant values), respectively at ionic strength 0.1 Mol L-1 and a temperature of 35° C.
Corresponding author: mailto:brijtew@yahoo.com
INTRODUCTION
Metal complexes play an important role in various biological systems [1] and in different field of chemistry [2]. Alloys of beryllium with copper and metal have excellent electrical and thermal conductivities. For this reason these alloys are used for fabricating aircrafts, missiles, spacecraft and communication satellites. When inhaled as a particulate, some beryllium compounds can lead to an irreversible and sometime fatal scarring of the lungs known as beryllosis or Chronic Beryllium Disease (CBD). CBD may also affect other organs, such as the lymph, nodes, skin, spleen, liver, kidneys and heart. Short-term beryllium exposure may lead to inflammation, reddening or swelling of the lungs, a condition known as Acute Beryllium Disease (ABD). Cobalt is a important component of vitamin B12. Normal amounts of cobalt in the diet around 1.5 mcg/day (micrograms per day). Sources of dietary cobalt found abundantly in all foods that are rich in vitamin B12 such as meat, fish, shellfish, milk, liver. Lower levels are found in some mushrooms (such as shitake) and seaweeds, but not in fruits or vegetables, therefore vegetarian can easily become cobalt deficient. Long-term vitamin B12 deficiency can result in demyelization of large nerve trunks and the spinal cord, in reduced white blood cells, and in pernicious anemia. Beryllium and cobalt has several significant applications in biological systems [3-20]. 2-Amino 3-butenoic acid is a naturally occurring amino acids which do not occur in proteins. It has several significant applications in biological systems [21-27].
Kiso [28] has done comprehensive study on paper electrophoretic migration of metal complexes. The electrophoretic technique usually suffers from numbers of defects, e.g. temperature rise during electrophoresis, capillary flow on paper, adsorption and molecular sizing affect the mobility of charged moieties [29]. The technique described here is almost free from these vitiating factors. Communications [30-32] from our laboratory described a new method for the study of metal complexes. The ionophoretic mobility of metal spots had been used to study binary complexes. A search of literature indicated that few reports available on binary complexes of Co(II) with (-aminobutenoic acid but no report is available on Be(II) binary complexes with (-aminobutenoic acid. In view of this attempts were made to study these complexes. In addition, present work describes paper electrophoretic technique for the determination of nature and stability constants of Be(II) / Co(II) - (-aminobutenoic acid complexes.
RESULTS
Literature reveals that an ionic species of amino acids are the sole coordinating species in complex formation with metal ions [33, 34]. Hence, a metal ion spot on the paper strip show a variation in composition of different ionic species of the amino acids in the background electrolyte. So the mobility to metal ion spot would depend upon the pH of the background electrolyte. The plot of overall electrolyte speed of metal spot against pH gives a curve with a number of plateaus (Figure 3). A constant speed over a range of pH is possible only when a particular complex species is overwhelmingly formed. Thus, every plateau is indicative of formation of certain complex species. In the region of low pH, concentration of [CH2 = CH – CH (NH3+) COOH] species of (-aminobutenoic acid is maximum and this species is non-complexing. Beyond this range, metal ion spots have progressively decreasing mobility, complexation of metal ions should be taking place with anionic species of (-aminobutenoic acid whose concentration increases progressively with increase of pH.
Figure 3 reveals that Be(II) and Co(II) ions form their first complex movement toward negative electrode. Hence, one [CH2 = CH – CH (NH2) COO-] must have combined with Be(II) and Co(II) ions to give 1:1 - [Be{ H2C = CH – CH(NH2) COO}]+ and [Co{H2C = CH – CH(NH2) COO}]+ complex cations, respectively. With further increase of pH, mobility in both the metal ions decreases giving rise to third plateau with zero mobility that indicates its neutral nature. The third plateau in each case is due to 1:2, metal – ligand complex. Hence, two [CH2 = CH – CH(NH2) COO-], (-aminobutenoic acid anion to give, 1:2, [Be{[CH2 = CH – CH(NH2) COO-}2], and [Co {CH2 = CH – CH(NH2) COO-}2] complexes, respectively. Further increase of pH in case of both metal ions has no effect on the mobility of metal ions which indicates formation of only 1:1 and 1:2 metal complexes. In general, the complexation of metal ions with (-aminobutenoic acid anion may be represented as:
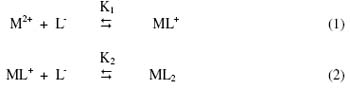
wherein M2+ is Be2+ and Co2+ metal ions; [L-] is (-aminobutenoic acid anion; K1 and K2 are first and second stability constants, respectively. The 1:1 and 1:2 metal complexes are formed at pH range 1.0 – 8.5. The metal spot on the paper is thus a combination of uncomplexed metal ions, a 1:1 complex and a 1:2 complex. The overall mobility is given by [35].

wherein u0, u1 and u2 are mobilities of uncomplexed metal ions, 1:1 metal complex and 1:2 metal complex, respectively.
For calculation of the first stability constant, K1 the region between the first and second plateau is pertinent. The overall mobility U will be equal to the arithmetic mean of the mobility of uncomplexed metal ion, u0, and that of first complex, u1 at a pH where K1 = 1/[H2C = CH – CH (NH2) COO-]. Using dissociation constant of pure (-aminobutenoic acid (electrophoretically obtained value, ka1 = 102.45; ka2 = 109.25), the concentration of the (-aminobutenoic acid anion [L-] is determined for the pH, from which K1 can be calculated. The concentration of chelating (-aminobutenoic acid anion [L-] is calculated with the help of equation.
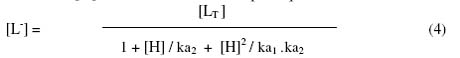
wherein [LT] = is the total concentration of ligand (-aminobutenoic acid; ka1 and ka2 are the first and second dissociation constants of pure (-aminobutenoic acid, respectively. The second stability constant K2, of the second complex can be calculated by taking into consideration the region between second and third plateau of the mobility curve. The calculated values of K1 and K2 are given in Table 1.
DISCUSSION
It is observed from Table 1 that values of first and second stability constant of binary complexes follow the order:
beryllium(II) > cobalt(II)
The values of second stability constant of ML2 complexes are found to be lower in comparison to first stability constant of ML complexes in each case this may be due to the decrease in coordinating tendency of ligand with the higher state of aggregation [36, 37]. High stability constant values of beryllium(II) - (-aminobutenoic acid complex indicate strong bonding between beryllium(II) ion and (-aminobutenoic acid, while low stability constant value of cobalt(II) - (-aminobutenoic acid complex indicate weak bonding between cobalt(II) cation and (-aminobutenoic acid anion.
According to Standard Deviation (statistics), the precision of the method is limited to that of paper electrophoresis, and uncertainty in the result is ± 5 %. Hence, it can not immediately replace the most reliable methods, even though it is new approach is worth further development.
To examine the possibility of hydrolysis of Be(II) at higher pH, experiments have been performed at two concentrations of the ligand: 0.01 Mol L-1 and 0.001 Mol L-1. The mobility curves show that the plateaus at lower ligand concentration are shifted towards higher pH range, but the calculated stability constants are found to be the same in the two cases. Thus the constant obtained is independent of the pH indicating that hydrolysis of Be(II) can be ignored here.
The probable structure for ML2 complex may be given as:
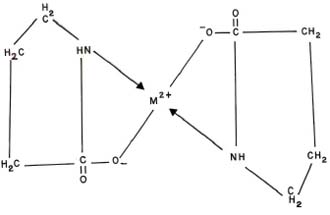
Table 1. Stability constants of binary complexes of beryllium(II) and cobalt(II) with 2 – amino – 3 - butenoic acid.
EXPERIMENTAL SECTION
Apparatus
A Systronic (Naroda, India) Model 604, electrophoresis system was used. The apparatus consisted of a PVC moulded double tank vessel. In our laboratory significant change in the instrument has been made. Two hollow rectangular plates covered with thin polythene sheets have been used through which thermostated water is run for controlling the temperature. The tanks are closed with a transparent PVC moulded lid. The whole assembly is tight which prevents moisture changes, which might upset the equilibria in the paper strip. The assembly design thus keeps to a minimum the disturbing effect of evaporation from the unwanted liquid flow in the paper strips. Each electrolyte tank contains a separate electrode chamber in which anode and cathode are placed, respectively. Electrophoresis cell showing sandwiched paper strips is shown in Figure 1.
Elico (Hyderabad, India,) Model L1-10 having glass and calomel electrode assembly and working on 220 V/50 Hz established a.c. mains, was used for the pH measurements
Chemicals
Beryllium(II) and cobalt(II) metal perchlorate solutions were prepared by the precipitation of metal carbonates from their nitrates with sodium carbonate, which were washed with boiling water and treated with calculated amounts of 1% perchloric acid. These were heated and filtered. The metal contents of the filtrates were determined and final concentration was kept at 0.005 Mol L-1. A 0.1% (w/v) solution of 1-(2pyridylazo) – 2 – naphthol (PAN) (Merck, Darmstadt, Germany) in ethanol was used for detecting the metal ions. 0.005 Mol L-1 glucose (BDH, AnalaR) solutions was prepared in water and used as an electro-osmotic indicator for the correction due to electro-osmosis. A saturated aqueous solution (0.9 ml) of silver nitrate was diluted with acetone to 20 ml. Glucose was detected by spraying with this silver nitrate solution and then with 2% ethanolic solution of sodium hydroxide, when a black spot was formed. Paper strips showing position of metal ions after electrophoresis is shown in Figure 2.
Background Electrolytes
Stock solution of 5.0 Mol L-1 perchloric acid was prepared by its 70% solution (SDS, AnalaR grade), 2.0 Mol L-1 sodium hydroxide (AnalaR grade) and 0.5 Mol L-1 (-aminobutenoic acid (BDH) solutions were prepared. Each solution was standardized using an appropriate method.
The background electrolyte used in the study of binary complexes were 0.1 Mol L-1 perchloric acid and 0.01 Mol L-1 (-aminobutenoic acid. The binary system was maintained at various pHs by the addition of sodium hydroxide.
Sodium hydroxide solution
Carbon dioxide free sodium hydroxide was prepared by dissolving 500 grams of sodium hydroxide in 500 ml of water in a flask. The flask was left overnight. The clear supernatant liquid was filtered rapidly through a sintered glass crucible using a high vacuum pump. A suitable volume of the filtrate was diluted and the concentration determined by titrating against a standard oxalic acid solution, and a solution (2.0 Mol L-1) was obtained by suitable dilution. The concentration of stock solution was checked from time to time.
Procedure
The hollow base plate in the instrument was verified to be horizontal with sprit level. 150 ml volume of background electrolyte was placed in each tank of the electrophoretic apparatus. The levels of the two tank solutions were equalized by a siphon. These precautions were taken to stop any gravitational and hydrodynamic flow. Paper strips (Whatman No. 1) of 30 x 1 cm2 size were soaked in background electrolyte and then excess of electrolyte solution was blotted by pressing them gently within the folds of dry filter paper sheets. Paper strips in duplicate were spotted with metal ions and glucose in the centre with a micropipette and were subsequently placed on the base plate and sandwiched under the upper hollow metallic plate with the ends of the strips immersed in the tank solutions on both side. Then a potential difference of 200V was applied between the tank solutions and electrophoresis was carried out for 60 minutes. Afterward the strips were taken out with the help of glass rod and dried. The spots were developed. The distance recorded in duplicate strips differed ± 5% and the average distance was calculated. The distance travelled towards anode were given a negative sign and those towards cathode to be positive. The actual distance of the sample spot moved was corrected for the distance travelled by the reference glucose spot. The mobilities were calculated by dividing the movement by the potential gradient and expressed in Cm2 V-1 min -1
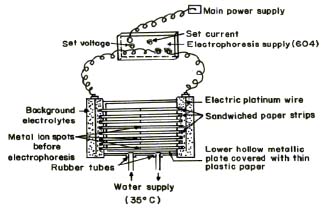
Fig.1 Electrophoresis cell showing sandwiched paper strips

Fig.2 Paper strips showing position of metal spots after electrophoresis

Fig. 3 Mobility curve for the metal(II) - (-aminobutenoic acid systems. = Be(II) - (-aminobutenoic acid = Co(II) - (-aminobutenoic acid. Background electrolytes = 0.1 Mol L-1 perchloric acid and 0.01 Mol L-1 (-aminobutenoic acid. Concentration of Be2+ and Co2+ = 0.005 Mol L-1. Ionic strength = 0.1 Mol L-1, temperature = 35ºC. The paper strips were spotted with 0.1 µl of sample solutions and glucose (for making osmotic corrections).
REFERENCES
1.SIEGEL, H. Metal Ions in Biological Systems, Vols 1 – 23, 1989, Marcel Deeker, New York. [ Links ]
2.SHERMAN S. E., LIPPARD S. J. Chem. Rev.,1987, 87, 1153. [ Links ]
3.ALDRIDGE, W. N. Ann. Rev. Pharmacol. Toxicol., 1986, 26, 39. [ Links ]
4.CHIARAPPA – ZUCCA, M. L., FINKEL, R. C., MARTINELLI, R. E., MCANINCH, J. E., NELSON, D. O., TURTELTAUB, K. W. Chem. Rev. Toxicol., 2004, 17(12), 1614. [ Links ]
5.KRAM, P., HRUSKA, J., DRISCOLL, C. T. Water, Air, Soil Pollu., 1998, 105, 409. [ Links ]
6.OLIVEIRA, K. A., VANNUCCI, A., GALVAO, R. M. O., ARAUJO, M. S. T., NASCIMENTO, I. C. Braz. J. Phys., 1998, 28(3), 230. [ Links ]
7.PAVLOR, M., SIEGBAHN, P. E. M., SANDSTROM, M. J. Phys. Chem. A, 1998, 102, 219. [ Links ]
8.SU, Y., JIN, Z., DUAN, Y., KOBY, M., MAJIDI, V., OLIVARES, J. A., ABELM, S. P. Anal. Chim. Acta, 2000, 422, 209. [ Links ]
9.BURASTERO, S. M. Chem. Res. Toxicol., 2003, 16(9), 1145. [ Links ]
10.KUSAKA, Y., SATO, K., SUGANUMA, N., HOSODA, Y. J. Occup. Health, 2001, 43, 1. [ Links ]
11.CEDARBRANT, K., GUNNARSSON, L. G., HULTMAN, P., NORDA, R., TIBBLING – GRAHN, L. J. Deut. Res. 1999, 78, 1450. [ Links ]
12.MARTIN, H. G., IVANOVA, N., KUNIN, V., HUGNHALTZ, P. Nature Biotechnol., 2006, 24, 1263. [ Links ]
13.WOOD, J. M. Science, 1974, 183 (4129), 1049. [ Links ]
14.EGAMI, F. J.Biochem., 1975, 77, 1165. [ Links ]
15.KAYE, P. T., NYOKONG, T., WATKINS, G. M., WELLINGTON, K. W. ARKIVOC, 2002, p, 9. [ Links ]
16.ALLYU, A. O., NWABUEZE, J. N. Int. J. Phys. Sci., 2008, 2(7), 167. [ Links ]
17.TODOROVSKA, N., KARADJOVA, I., ARPADIAN, S., STAPILOV, T. Acta Pharma., 2003, 53, 83. [ Links ]
18.MILES, A. T. HAWKSWORTH, G. M. BEATTIE, J. H., RODILLA, V. Critical Rev. Biochem. Mol. Biol., 2000, 35(1), 35. [ Links ]
19.OSINSKY, S., LEVITIN, I., BUBNVSKAYA, L., SIGAN, A., GANUSEVICK, I., WARDMAN, P. Exp. Oncol, 2004, 26(2), 140. [ Links ]
20.LINNA, A., OKSA, P., GROUNDSTROLM, K., HALKOSAARI, M., PALMROOS, P., HUIKKO, S., UIM, J. Occup. Environ. Med., 2004, 61, 877. [ Links ]
21.THORUBERRY, N. A., BULL, H. G., TAUB, D., WILSON, K. E., GIMENEZ-GALLEGO, G., ROSEGAY, A., SODERMAN, D. D. J. Biol. Chem., 1991, 266(32), 21657. [ Links ]
22.MITCHELL, R. E., Cellu. Mol. Life Sci., 1991, 47, 791. [ Links ]
23.SAWADA, S., NAKAYAMA, T., ESAKI, N., TANAKA, H., SODA, K. HILL, R. K., J. Org. Chem., 1986, 51, 3384. [ Links ]
24.FREDENHAGEN, A., MOLLEYRES, L. –P., BOHLENDORF, B., LAVE, G. J. Antibiot., 2006, 59(5), 267. [ Links ]
25.ELICH, T. D., LAGARIAS, J. C. Plant Physiol., 1988, 88, 747. [ Links ]
26.KABAYASHI, K., IRISAWA, S., AKAMATSU, H., TAKAHASHI, M., KITAMURA, T., TANMATSU, M., MORIKAWA, T., TANMATSU, M., Bull. Chem. Soc. Jpn., 1999, 72 (10), 2307. [ Links ]
27.SILVERMAN, R.,B., LEVY, M. A., J. Biol. Chem., 1981, 256(22), 11565. [ Links ]
28.KISO, Y., Electrophoresis, New Attempts of Ionics, 1972, Nankodo, Japan. [ Links ]
29.MCDONALD, H. J. Ionography, Electrophoresis in Stabilized Media, 1975, Year Book Publications, Chicago, USA. [ Links ]
30.TEWARI, B. B., KAMALUDDIN, SRIVASTAVA, S. K., J. Indian Chem. Soc., 1996, 73, 75. [ Links ]
31.TEWARI, B. B., Russ. J. Inorg. Chem., 1994, 30(12), 1950. [ Links ]
32.TEWARI, B. B., SINGH, R. K. P., YADAVA, K. L., Bull. Soc. Chim. Fr. 1991, 128, 141. [ Links ]
33.HOJO, Y., SUGIURA, Y., TANAKA, H., J. Inorg. Nucl. Chem., 1977, 39, 1859. [ Links ]
34.WALKER, D. M., WILLIAMS, R. D., J. Chem. Soc. Dalton, 1974, 1186. [ Links ]
35.NICHOL, J., J. Am. Chem. Soc., 1950, 72, 2367. [ Links ]
36.JOSHI, J. D., BATTACHARYA, P. K., J. Indian Chem. Soc., 1973, 50, 344. [ Links ]
37.JOSHI, J. D., BHATTACHARYA, P. K., J. Indian Chem. Soc., 1980, 57, 336. [ Links ]
38.MARTELL, A. E., SMITH, R. M. Critical Stability Constants, Vol. 1 Amino Acids, 1974, Plenum Press, New York and London, p. 14. [ Links ]











 uBio
uBio