Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Boliviana de Química
On-line version ISSN 0250-5460
Rev. Bol. Quim vol.26 no.1 La Paz Aug. 2009
ARTICULO ORIGINAL
COMPLEX FORMATION OF SOME DIVALENT METAL IONS WITH OXYGEN DONOR LIGANDS
Brij B. Tewari
Department of Chemistry, Faculty of Natural Sciences, University of Guyana, P. O. Box: 101110, Georgetown, Guyana, Tel: 592-222-6002; Fax: 592-222-3596
Keywords: Paper electrophoretic technique; overall mobility; beryllium(II) complexes; cobalt(II) complexes; a-aminobutyric acid, stability constant.
ABSTRACT
Complexation reaction of a-aminobutyric acid with beryllium(II) and cobalt(II) have been studied in solution phase using paper electrophoretic technique. This method is based on the movement of a spot of metal ion in an electric field at various pH’s of the background electrolyte. A plot of overall mobility of metal / complex ion versus pH was used to obtain information on the binary complexes and to calculate stability constants. The stability constant of the ML and ML2 complexes of beryllium(II) - a-aminobutyric and cobalt(II) - a-aminobutyric acid have been found to be (7.19 ± 0.05, 6.81 ± 0.13) and (4.47 ± 0.04, 2.81 ± 0.09) (logarithm stability constant values), respectively at 35°C and ionic strength 0.1 M.
Corresponding author: brijtew@yahoo.com
INTRODUCTION
For a mononuclear binary complex, if a central atom (central group) M (the ‘metal’) and a ligand L have been defined, then in the following expressions Kn is the stepwise formation constant, and βn is the cumulative formation constant for the complex MLn. They can both be referred to as stability constants (stepwise and cumulative) [1].Kn = K (MLn-1 + L = MLn)
βn = K(M + nL = MLn)
Metal complexes play an important role in various biological systems, hence the formation, stability and reactivity of these complexes have been an active field of research [2,3]. The inhalation of beryllium compounds lead to a fatal scarring of the lungs known as Chronic Beryllium Disease (CBD). CBD is treatable, but not curable. Treatment may involve use of steroids to reduce inflammation, which may slow the progress of CBD by reducing the buildup of scar tissue and delaying permanent lung damage. The common symptoms of CBD include shortness of breath upon exertion, weight loss, cough, fatigue, chest pain, anorexia, and overall weakness. Immunological and genetic influences on development of CBD have been identified. Cobalt is an integral part of vitamin B12. Cobalt can be toxic to humans when consumed in excessive quantities. Excesses can cause polycythemia, bone marrow hyperplasia, pancreatic failure or congestive heart failure and cardiomyopathy. The synthetic isotope 60Co is used extensively as a tracer and in cancer radiotheraphy. Long – term vitamin B12 deficiency can result in demyelination of large nerve trunks and the spinal cord, in reduced white blood cells, and in pernicious anemia. Foods containing cobalt include meat, milk, eggs, fish and cabbage. Cobalt supplements may be necessary for strict vegetarian diets. Beryllium and cobalt ions are well known for their biomedical application and toxicity. [4-20]. a-aminobutyric acid is a naturally occurring amino acids which do not occur in proteins. It is found in animal and plant tissues. It has significant application in biological systems [21-28]. Kiso [29] has done comprehensive study on paper electrophorectic migration of metal complexes.
The Paper Electrophoretic Technique usually suffers from a number of defects. Temperature during electrophoresis, capillary flow on paper, electro-osmosis and adsorption affect the mobility of charged moieties [30]. The present technique is almost free from these destroying factors and very convenient in use. It gives results in fair agreement with accepted literature values. Publications [31-33] from our laboratory described a new method for the study of metal complexes. A search of literature indicated few reports on Cu(II) - a-aminobutyric acid complex but no report on Be(II) -a-aminobutyric acid complex. In view of this, attempts were made to establish the optimum conditions for metal(II) - a-aminobutyric acid complex formation. In addition, present paper describes on paper electrophoretic method for the determination of the nature and stability constants of Be(II)/Co(II) - a-aminobutyric acid complexes.
RESULTS
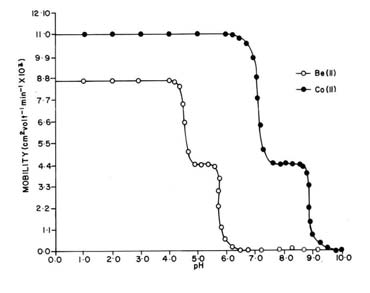
Literature reveals that an ionic species of amino acids are the sole coordinating species in complex formation with metal ion [34, 35]. Hence a metal ion spot on the paper strip show a variation in composition of different ionic species of the amino acids in the background electrolyte. So the mobility of metal ion spot would depend upon the pH of the background electrolyte. Mobility of metal ion complexes against pH gives a curve containing three plateus in each case of Be2+ and Co2+ metal ions (Figure 1). The first plateau in each case at low pH region, represents uncomplexed metal ion, while the remaining ones indicate metal complexes. In the low pH range a-aminobutyric acid is present as a non-complexing species [CH3 – CH2 – CH (NH3+) COO-]. Beyond this range metal ion spots have progressively decreasing mobility and hence complexation of metal ions should be taking place with anionic species of a-aminobutyric acid [CH3 – CH2 – CH (NH2) COO-], the concentration of which increases progressively with increase of pH.
Fig. 1. Mobility curve for the metal(II) - a-aminobutyric acid systems. ![]() = Be(II) - a-aminobutyric acid
= Be(II) - a-aminobutyric acid ![]() = Co(II) - a-aminobutyric acid. pHs were maintained by addition of sodium hydroxide. Ionic strength = 0.1 M, temperature = 35ºC. The paper strips were spotted with 0.1 µl of sample solutions and glucose (for making osmotic corrections).
= Co(II) - a-aminobutyric acid. pHs were maintained by addition of sodium hydroxide. Ionic strength = 0.1 M, temperature = 35ºC. The paper strips were spotted with 0.1 µl of sample solutions and glucose (for making osmotic corrections).
Figure 1 reveals a second plateau in each case with positive mobility indicating the formation of 1:1 complex of cationic nature. Further increase of pH gives rise to a third plateau with zero mobility in each case indicating the formation of 1:2 complexes of neutral nature. This is possible only when two anionic species of a-aminobutyric acid combined with divalent metal cations. In general, the complexation of metal ions with a-aminobutyric acid anion may be represented as

wherein M2+ is Be2+ and Co2+ metal ions; [L-] is a-aminobutyric acid anion [CH3 – CH2 – CH (NH2) COO-]; K1 and K2 are the first and second stability constants, respectively.
The metal spot on the paper is thus a combination of uncomplexed metal ions, 1:1 and 1:2 metal complexes. The spot is moving under the influence of electric field, the overall mobility is given by equation of Jokl [36].
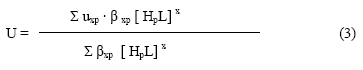
Wherein [HpL]x is the concentration of general complex species, βxp is the overall mobility constant of the complex; Uxp is the speed of the general complex [M (HpL)x] present in the combination. On taking into consideration different equilibria, the above equation is transformed into the following form.
U0 + u1 K1 [L-] + U2 K1 K2 [L-]2
U = _____________________________ (4)
1 + K1 [L-] + K1 K2 [L-]2
Wherein u0, u1 and u2 are mobilities of uncomplexed metal ions, 1:1 metal complex and 1:2 metal complex, respectively. Eq. (4) was used for the determination of the stability constants of metal complexes with a-aminobutyric acid.
The dissociation constant of pure a-aminobutyric acid (k1 = 10 2.30; k2 = 109.63) was determined by the same paper electrophoretic technique. The mode of dissociation of pure a-aminobutyric acid may be represented as:
[CH2 (OH) CH2 CH (NH3+) COOH]
-H+ E ka1
[CH2 (OH) CH2 CH (NH3+) COO-]
-H+ E ka2
[CH2 (OH) CH2 CH (NH2) COO-]
For calculating first stability constant, K1 the region between first and second plateau is relevant. The overall mobility will be equal to the arithmetic mean of the mobility of uncomplexed metal ion, u0, and that of first complex, u1 at a pH where K1 = 1/[L-].
First stability constant K1, can be calculated with the help of concentration of homoserine anion and protonation constant of pure homoserine. The concentration of ligating homoserine species [L-] is calculated with the help of equation.
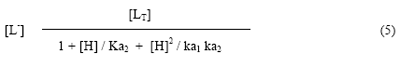
Where [LT] = is the total concentration of ligand homoserine (0.01 M); k1 and k2 are the dissociation constants of pure a-aminobutyric acid, respectively. The second stability constant K2, of 1:2 complex can be calculated by taking into consideration the region between second and third plateau of the mobility curve. The calculated values of first and second stability constants are given in Table 1.
Table 1. Stability constants of binary complexes of beryllium(II) and cobalt(II) with a-aminobutyric acid.
| Metal Ions | Complexes | Stability constants | Logarithm stability constant values |
| Beryllium(II) | ML+
ML2 | K1
K2 | 7.19 ± 0.05
6.81 ± 0.18 |
| Cobalt(II) | ML+
ML2 | K1
K2 | 4.47 ± 0.04 4.26 [41]* 4.21 [42]* 2.81 ± 0.09 3.42 [41]* 3.50 [42]* |
Ionic strength = 0.1 M; temperature = 35ºC; a-aminobutyric acid = [CH3 – CH2 – CH (NH2) COO-]; M = metal cations; L = ligand (a-aminobutyric acid); * = literature values.
DISCUSSION
It is clear from Table 1 that first and second stability constants of both beryllium(II) and cobalt(II) complexes follow the order.
Log K1 > log K2
The corresponding second stability constant values are found to be lower for both complexes. It is therefore inferred that coordinating tendency of a ligand decreases with the higher state of aggregation [37,38].
It is also clear from Table 1 that beryllium(II) - a-aminobutyric acid complexes have high stability constant value in comparison to cobalt(II) – a-aminobutyric acid. Therefore it can be inferred that beryllium(II) cation has greater affinity with oxygen donor ligand in comparison to cobalt(II) anion.
The stability constants of metal complexes can be very easily calculated by this technique, therefore present method has significant advantages over other methods (viz: polargraphic, potentiometric, solubility etc.) reported in chemical literature for the determination of stability constants of metal complexes.
The present paper ionophoretic technique is limited to charged species and the precision of method is not as which as other physiochemical methods. However, uncertainty in the result is ±5%. No doubt it can not replace the most reliable methods even though it is a new approach deserving further development.
To examine the possibility of hydrolysis of beryllium(II) at higher pH, experiments have been performed at two concentrations of the ligand: 0.01 M and 0.001 M. The mobility curves show that the plateaus at lower ligand concentration are shifted towards higher pH range, but the calculated stability constants are found to be the same in the two cases. Thus the constant obtained is independent of the pH indicating that hydrolysis of beryllium(II) can be ignored here.
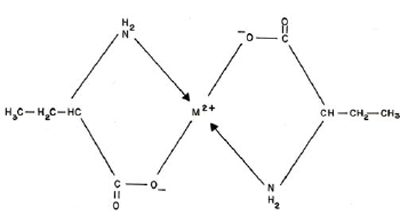
The proposed structure for the ML2 complexes may be given as:
EXPERIMENTAL SECTION
Instruments
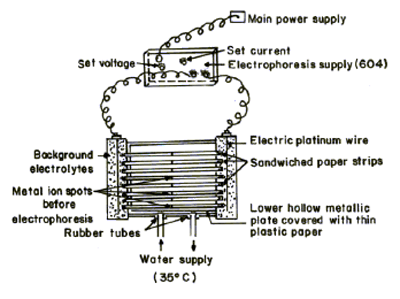
Systronics (Naroda, India) paper elelctrophoresis equipment horizontalcum-vertical type, model 604, has been used. The apparatus consisted of a PVC moulded double tank vessel. In our laboratory significant changes in the instrument has been made. Two hollow rectangular plates covered with think polythene sheets have been used through which thermostated water is run for controlling the temperature. The tank is closed with a transparent PVC moulded lid. The whole assembly is tight, which prevent moisture changes, which may upset the equilibria in a paper strip. This assembly design thus keeps to a minimum the disturbing effects of evaporation from the unwanted liquid flow in the paper. Each electrolyte tank contains a separate electrode chamber. The auxiliary unit is specially designed to operate either voltage mode or on current mode.
Elico (Hyderabad, India) model L1-10 having glass and calomel electrode assembly and working on 220 Volts/ 50 Hz established a.c. mains, were employed for pH measurements. The electrophoresis cell showing sandwiched paper strips and water supply is shown in Figure 2.
Chemicals
Water
Distilled water was redistilled over alkaline permanganate. The resulting distillate cooled in well Stoppard Pyrex flask. This was used for preparing solutions and for dilution throughout their studies.
Metal Solutions
Metal perchlorate solutions were prepared by the precipitation of metal carbonates from their nitrates with sodium carbonate, which were washed with boiling water and treated with calculated amounts of 1% perchloric acid. There were heated and filtered. Beryllium perchlorate solution was standardised by gravimetric method. 25 ml of beryllium perchlorate was taken in beaker and to it ammonia solution was added dropwise until the hydroxide commence to precipitate. Redissolved the precipitate by adding a few drops of 0.1 M hydrochloric acid. Approximate 1 mg of ammonium chloride, sufficient amount of EDTA solution was added to it to complex all other trace metal ions (if present). Again a little amount of ammonia solution was added to it and the contents filtered through a gravimetric filter paper. Placed the paper on a silica crucible, dried, and then heated it by a flame throw over. Cooled the crucible in a coved magnesium perchlorate and weighed immediately when cooled. Cobalt(ii) perchlorate solution standardized by volumetric method. Cobalt(II) solution was taken in a conical vessel to which standard EDTA solution was added in known excess and to it were then added 5 ml buffer pH 5, and 3-5 drops of 1-(2-pyridylazo-2-naphthol (PAN indicator). The solution was diluted to 60 ml. The unused EDTA was titrated against standard copper(II) perchlorate solution until the colour changed first to violet. A few drops of the EDTA solution was then added to restore the yellow colour . The amount of Co(II) was known from the amount of EDTA consumed by it. The metal contents of the filtrates were determined and final concentration was kept at 5.0 x 10-3 M [39,40].
Detecting reagents for metal ions and electro-osmotic indicator
A 0.1% (w/v) solution of 1 – (2 – pyridylazo) – 2 – naphthol (PAN) (Merck, Darmstadt, Germany) in ethanol was used for detecting the metal ions. 5.0 x 10-3 M glucose (BDH, AnalaR) solutions was prepared in water and used as an electro-osmotic indicator for the correction due to electro-osmosis. A saturated aqueous solution (0.9 ml) of silver nitrate was diluted with acetone to 20 ml. Glucose was detected by spraying with this silver nitrate solution and then with 2% ethanolic sodium hydroxide, when a black spot was formed. The paper strips showing position of metal ion spots after electrophoresis is shown in Figure 2.
Fig. 2 Electrophoresis cell showing sandwiched paper strips.
Background electrolyte
Stock solution of 5.0 M perchloric acid was prepared by its 70% solution (SDS, Analytical – Reagent grade), 2.0 M sodium hydroxide (Analytical – Reagent grade) and 0.5 a-aminobutyric acid (BDH, Poole, UK) solutions were prepared. Each solution were standardized using an appropriate method. The background electrolyte used in the study of binary complexes were 0.1 M perchloric acid and 0.01 M a-aminobutyric acid. The binary system was maintained at various pHs by the addition of sodium hydroxide.
Procedure
Whatman No. 1 filter paper for chromatography was used for the purpose of electrophoresis. For recording observation of particular metal ion, two paper strips were spotted with the metal ion solution along with additional two spotted with glucose using 1.0 µl pipette and then mounted on the insulated plate. Each of the two electrolyte vessels were filled with 150 ml of background electrolyte containing 0.1 M perchloric acid and 0.01 M a-aminobutyric acid.
The paper becomes moistened with the background electrolyte solutions due to diffusion. The second insulated plate was placed on paper strips and then thermostated water (350C) was circulated into the plates to keep the temperature constant. The lid was then placed on the instrument to make it air tight. It was left for 10 minutes to insure wetting of strips. Subsequently a direct 220 V potential was applied between the electrodes. Electrophoresis was carried out for 60 minutes after with the strips were removed from the tank and dried. The metal ion and glucose spots were detected by specific reagents. The leading and tailing edge were measured from marked center point and the mean taken. The distance moved by glucose spot was subtracted (in case of migration toward anode) to obtain correct path length. Migration towards anode and cathode were designated by negative and positive signs respectively.
Electrophoretic observation of metal ions were recorded at various pH values of the background electrolyte obtained by adding NaOH solution, the ionic strength being maintained at 0.1 M. The observed mobility of migrant was calculated by using the formula:
d
U =_______________
x · t
After applying the correction factor the observed mobility is given as:
d ± dG
U =__________________
x · t
Where U = mobility of metal ion / complex ion; d = mean of duplicate distance travelled by metal ion / complex ion; dG = mean of duplicate distance travelled by glucose spot; x = field strength; t = time for electrophoresis.
The protonation constants of pure a-aminobutyric acid were determined by the same paper electrophoretic technique. The two paper strips were spotted with pure a-aminobutyric acid along with two other spotted glucose using 0.1 M perchloric acid only as a background electrolyte. The electrophoresis was carried out for 60 minutes as for metal ions. The electrophoretic speed was calculated.
The speeds of the metal ion/amino acid are reported with pH values. The individual speeds of the duplicate spots were found to be fairly equal. A plot of mobility against pH is shown in Figure 3.
Fig. 3 Paper strips showing position of metal ion spots after electrophoresis
CONCLUDING REMARKS
Following conclusion can be drawn from the present study.
1.Beryllium(II) and cobalt(II) are toxic and essential metal ions respectively.
2.Beryllium(II) and cobalt(II) are significant for biological systems but as such they are toxic, the a-aminobutyric acid may be used to reduce the level of these metal ions in the biological system.
3.The ML2 complexes are found to have low stability constants values and less stable in comparison to ML complexes.
4.Beryllium(II) - a-aminobutyric acid complexes are found to have high stability constant values in comparison to cobalt(II) - a-aminobutyric acid complexes.
5.The present simple electrophoretic technique has thus proved to be helpful in deciding whether a complex system is formed or not, and if it is formed its stability can also be determined.
6.Biologically important beryllium(II) and cobalt(II) complexes with a-aminobutyric acid can be prepared on large scale at particular pH of background electrolyte solution.
REFERENCES
1. IUPAC, Compendium of Chemical Terminology, O. B. 11, 2nd Edition, 1997.
2. SHERMAN S. E., LIPPARD S. J., Chem. Rev. 87 1153. [ Links ]
3. BHATTACHARYA, P. K., J. Sci. Ind. Res., 1981, 40, 382. [ Links ]
4. ALDERIGHT, L., GANS, P., IENCO, A., PETERS, D., SABATINI, A., VACCA, A., Coord. Chem. Rev., 1999, 184 , 311. [ Links ]
5. FRANKEL, R. D., FORSYTH, J. M., Science, 1979, 204, 622. [ Links ]
6. JAKUBOWSKI, M., TRZCINKA-OCHOCKA, M., J. Occup. Health, 2005, 47 (1), 22. [ Links ]
7. PANESSA – WARREN, B., J., WARREN, J. B., WONG, S. S., MISEWICH, J.A., J. Phys: Condens, Matter, 2006, 18, 52185.
8. KLEIN, R., SCHWENK, M., HEINRICH – RAMM, R., TEMPLETON, D. M., Pure Appl. Chem., 2004, 76 (6), 1269.
9. GILI, P., MEREROS, A., Rev. Soc. Quim. Mexico, 2000, 44(1), 104. [ Links ]
10. KANAI, T., ENDO, M., MANOHARA, S., MIYAHARA, N., KOYAMA-ITO, H., TOMURA, H., MITSUFUJI, N., FUTAMI, Y., FUKUMURA, A., HIRAOKA, T., FURUSAWA, Y., ANDO, K., SUZUKI, M., SUGA, F., KAWACHI, K., Int. J. Radiat. Oncol. Biol. Phys., 1999, 44(1), 201. [ Links ]
11. WITSCHI, H. P., ALDRIDGE W. N., Biochem J., 1968, 106, 811. [ Links ]
12. RAMOS, R.G., OLDEN, K., Int. J. Environ. Res. Public Health, 2008, 5(1), 4. [ Links ]
13. EGGEMAN, A. S., PETFORD-LONG, A. K., DOBSON, R. J., WIGGINS, J., BROMWICH, T., DUMIN-BORKOWSKI, R., KASAMA, T., J. Magn. Magn. Mater., 2006, 301, 336. [ Links ]
14. ATWATER, J. E., ARSE, J. R., JOBANOVIC, G. N., WHEELER, R. R., SORNCHAMNI, T., Mater. Res. Bull. 2003, 38, 395. [ Links ]
15. GAD, N., Res. J. Agri. Biol. Sci, 2005, 1(3), 270. [ Links ]
16. KOVALA – DEMERTZI, D., HADJIKAKOU, S. K., DEMERTZIS, M.A., DELIGIANAKIS, Y., J. Inorg. Biochem., 1998, 69(4), 223.
17.WALSH, C., BEGLEY, T., WALTS, A., Pure & Appl. Chem., 1987, 59(3), 295. [ Links ]
18. STEVENS, H., JANSEN, H. M. L., DEREUCK, J., LEMMERLING, M. A., STRIJCKMANS, K., GOETHALS, P., LEMAHIEU, I., DE JONG, B. M., ILLEMEEN, A. T. M. W., KORF, J., J. Neurol. Sci., 1999, 171, 11. [ Links ]
19. BENZAMIN-III, E., REZNIK, A., BENZAMIN, E., WILLIAMS, A. L., Int. J. Environ. Res. Public Health, 2007, 4(3), 203. [ Links ]
20.EMAN, A., GAD, N. BADRAN, N. W., Aust. J. Basic Appl. Sci., 2007, 1(2), 73. [ Links ]
21.VUCKOVIC, G., TANASKOVIC, S. B., MIODRAGOVIC, S. M., STANIC, V., J. Serb. Chem. Soc. 2007, 72(2), 1295. [ Links ]
22.JOHNSON, T. W., KOSTIC, N. M., J. Serb. Chem. Soc. 2004, 69(4), 887. [ Links ]
23.MICKELSON, J. W., JACOBSEN, E. J., CARTER, D. B., Im, H. K., SCHREUR, R. J., SETHY, V. H., TANG, A. H., McGHEE, J. E., PETKE, J. D., J. Med. Chem. 1996, 39, 4654. [ Links ]
24.YIN, M., SVED, A. F., Hypertension, 1996, 27, 1291. [ Links ]
25.FUHRMAN, J. A., BELL, T. M., Mar. Ecol. Prog. Ser., 1985, 25, 13. [ Links ]
26.KISUMI, M., KATO, J., KOMATSUBARA, S., CHIBATA, J., Appl. Microbiol., 1971, 21(4), 569. [ Links ]
27.KEPPLER, O. T., HORSTKORTE, R., PAWLITA, M., SCHMIDT, C., REUTTER, W., Glycobiol, 2001, 11(2), 11 R. [ Links ]
28.KOROLKIEWICZ, R., SLIWINSKI, W., REKOWSKI, P., HALAMA-BOROWIEC, A., MUCHA, P., SZCZUROWICZ, A., KOROLKIEWICZ, K. Z., Pharmacol. Res., 1997, 35(1), 7. [ Links ]
29.KISO, Y., Zone Electrophoresis, New Attempts of Ionics, Nankodo, 1972. [ Links ]
30.McDONALD, H. J., Ionography, Electrophoresis in Stabilized Media, Year Book Publications, Chicago, 1975. [ Links ]
31.TEWARI, B. B., Trans. SAEST, 1995, 30(2), 76. [ Links ]
32.TEWARI, B. B., Trans. SAEST, 1995, 30(3), 100. [ Links ]
33.TEWARI, B. B., Bull. Korean Chem. Soc., 2002, 23(5), 705. [ Links ]
34.HOJO, Y., SUGIURA, Y., TANAKA,H., J. Inorg. Nucl. Chem., 1977, 39, 1859. [ Links ]
35.WALKER, D. M., WILLIAMS, R. W., J. Chem. Soc. Dalton, 1974, 1186. [ Links ]
36.JOKL, V., J. Chromatogr., 1964, 432. [ Links ]
37.JOSHI, J. D., Bhattacharya, P. K., J. Indian Chem. Soc., 1980, 57, 336. [ Links ]
38.JOSHI, J. D., Bhattacharya, P. K., J. Indian Chem. Soc., 1973, 50, 344. [ Links ]
39.KOLTHOFF, I. M., BELCHER, R., Volumetric Analysis, Vol. 3, 1957, Intersciences Publisher Inc., New York. [ Links ]
40.VOGEL, A. I., Text Book of Quantitative Inorganic Analysis: Including Elementary Instrumental Analysis, 4th Edition, 1978, Longmans, London. [ Links ]
41.PERRIN, D. D., Stability Constants of Metal Ion Complexes, Part B, Organic Ligands, IUPAC Series No. 22, 1979, Pergamon Press, Oxford: 313. [ Links ]
42.SILLEN, L. G., MARTELL, A. E., Stability Constants of Meal Ion Complexes, Special Publication No. 17, 1974, Chemical Society London:333