Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Boliviana de Química
On-line version ISSN 0250-5460
Rev. Bol. Quim vol.26 no.1 La Paz Aug. 2009
ARTICULO ORIGINAL
A 5-METHYLCOUMARIN GLUCOSIDE AND A COUMESTAN DERIVATIVE FROM MUTISIA ORBIGNYANA
Yonny Floresa,b, Gloria Rodrigoc,d, Patricia Mollinedoa, Bjorn Akessond, Olov Sternerb, Giovanna R. Almanzaa
a Instituto de Investigaciones Químicas, Universidad Mayor de San Andrés, C.P 303 La Paz, Bolivia,
b Division of Organic Chemistry, Lund University, PO Box 124, 221 00 Lund Sweden,
c Instituto de Biología Molecular y Biotecnología, Carrera de Biología, Universidad Mayor de San Andrés, La Paz, Bolivia,
d Biomedical Nutrition, Pure and Applied Biochemistry, Lund University, Lund, Sweden
Keywords: Mutisia orbignyana; Compositae; coumarins, antiproliferative capacity; scavenging effect; Bolivian medicinal plants.
ABSTRACT
The ethanolic extract of the aerial parts from Mutisia orbignyana afforded two major compounds: mutisifurocoumarin (1) and 5-methylcoumarine-4-β-glucoside (2). The completely assignment of 1H and 13C NMR data of compound (2) is presented for the first time as well as some reassignments of 13C NMR spectrum for compound (1), applying 2D NMR techniques. In addition, the antiproliferative effect on colon cancer cells (CaCo2) and the scavenging effect using the ABTS test were measured The results showed an interesting scavenging activity and a non proliferative effect on colon cancer cells./El extracto etanólico de las partes aéreas de Mutisia orbignyana presentó dos compuestos mayoritarios: mutisifurocumarina (1) y 5-metilcumarina-4-β-glucosilada (2). El asignamiento completo de RMN de 1H y 13C del compuesto (2) es presentado por primera vez, así como también algunos reasignamientos del espectro de RMN13C del compuesto (1), en ambos casos se aplico técnicas de RMN 2D. Además, se midió el efecto antiproliferativo sobre células cancerosas de colon (CaCo2) y el efecto como inhibidores de radicales libres usando la prueba ABTS, tanto de extractos como de compuestos puros. Los resultados muestran un interesante efecto antiradicalario en ABTS y un efecto no-proliferativo sobre células cancerosas de colon.
Corresponding author: giovyalmanza@yahoo.com.ar
INTRODUCTION
Bolivian highland plants, i.e. those which grow between 3 000 to 4 500 meters above sea level, are submitted to a high environmental stress which could have resulted in the production of secondary metabolites as a chemical mean of adaptation [1], [2]. In addition, many of these plants are used in the Bolivian traditional medicine. For this reason, Mutisia orbignyana Wedd. was selected for a study, as it grows at 3800 m.a.s.l. and is used in the traditional medicine of the region.
The South American genus Mutisia (Asteraceae, Compositae, tribe Mutisieae, subtribe Mutisiinae), with about 60 species is widely distributed from Colombia to Argentina and Chile [3]. Several of these species are used in South American folk medicine for the treatment of various diseases. For example, M. acuminata var. acuminata is used to treat cancer, gastric ulcers and respiratory diseases [4]; infusion of M. freisiana is used as a remedy against chronic coughs and stomach pains [5]; M. retrorsa cav. virreina is used to treat cancer [6]; M. viciaefolia to treat heart diseases, hysteria and epilepsy[6] and our plant in study, M. orbignyana known in Oruro - Bolivia as “Chilca”, is used in infusion to treat respiratory diseases and stomach pains.
Some Mutisia extracts have been submitted to pharmacological evaluations, and the antimicrobial activity of M. acuminata [7], [8], the radical scavenging activity of M. freisiana [9] and the anti-inflammatory activity of M. kurtzii [10] have been reported. Also chemical studies have been performed, and the isolation of flavonoids [9], [11], chromones [6], [9], [11], [12], sesquiterpenes [13], [14] and 5-methylcoumarins [3], [6], [12], [15] have been reported.
Previous studies of M. orbignyana have yielded several derivatives of 4-hydroxy-5-methylcoumarin [3]. In this paper we report the isolation of two other derivatives of 4-hydroxy-5-methylcoumarin: mutisifurocoumarin 1 and 5-methylcoumarin-4-β-glucoside 2. For both compounds a complete assignment of their 13C NMR data based on 2D NMR experiments is given. In addition, we present the radical scavenging evaluation using the ABTS [radical cation 2,2’-azino-bis(3-ethylbenzothiozoline-6-sulfonate)] test as well as their antiproliferative effect on colon cancer cells (CaCo2) for the raw extract as well as the pure compounds.
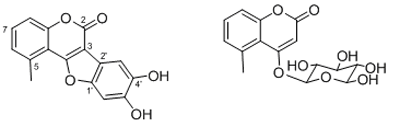
1 2
Figure 1. Major compounds from M. orbigniana
RESULTS, DISCUSSION
The recollected ethnopharmacological data of plants used in the Bolivian Highlands show that M. orbignyana, commonly known as “Chilca”, has a recognized use in the treatment of respiratory and gastric diseases. In addition, a preliminary antioxidant screening of Bolivian Highland plants pointed out an interesting antioxidant activity (86% I in ABTS) in the EtOH extract. Based on those data we selected this extract for further chromatographic fractionation obtaining the compounds 1 and 2 in pure form.
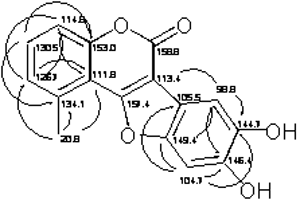
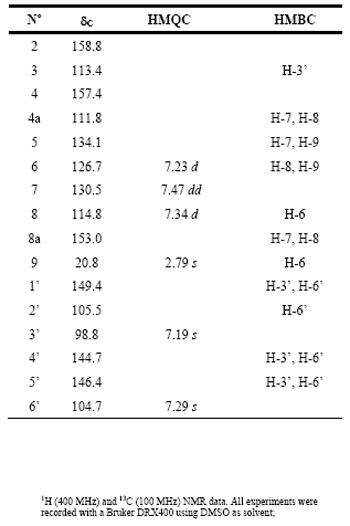
Compound 1: Mutisifurocoumarin was obtained as pale yellow crystals. High resolution mass spectrometry suggested that the elemental composition of 1 is C16H10O5, which is consistent with 1D NMR data (see Table 1). The 1D and 2D NMR data are in agreement with the proposal structure for 1 that was previously reported from two Mutisia species, first as 11,12-dihydroxy-5-methylcoumestan [6] and second, in its acetylated form, as mutisifurocoumarin diacetate [3]. The 1D NMR data are very close to those of the first report but the assignments of 13C NMR spectrum are quite different because the previous assignments were done using just 1D NMR data. In Table 1 the completely assignment of the 13C NMR data as well as the heteronuclear HMQC and HMBC data, suggesting the following changes: the signal at d 98.8 d should be assigned to C-3’ instead of C-6’ (C-13); d 104.7 d to C-6’ instead of C-3 (C-3); d 113.5 s to C-3 instead of C-5 (C-5); d 114.7 d to C-8 instead of C-3’ (C-10); d 126.6 d to C-6 instead of C-8 (C-8); d 134.1 s to C-5 instead of C-6 (C-6); d 144.8 s to C-4’ instead of C-1’ (C-14) and finally d 149.5 s to C-1’ instead of C-4’ (C-11), in parenthesis we show the original numbering used in reference [6]. HMBC correlation is showed in Figure 2.
Figure 2. Pertinent HMBC correlations observed with compound 1.
Table 1: NMR data for compound 1
Table 2. NMR data for compound 2
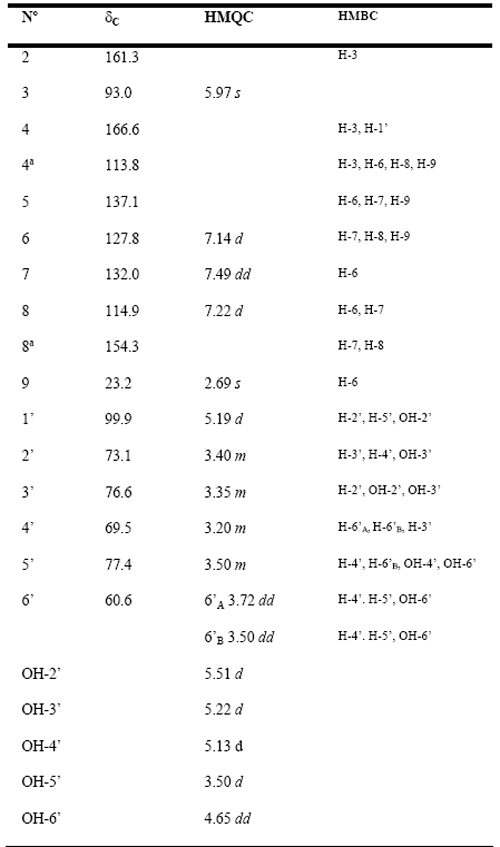
Compound 2: 5-methylcoumarine-4-b-glucoside is the major secondary metabolite of the EtOH extract. Its structure is consistent with the elemental composition C16H18O8 suggested by HREIMS (m/z 339.1067. Calcd. 339.1074) and was determined based on 1D and 2D NMR data. So, the 1H NMR spectrum revealed the characteristic signals of a 4-hydroxy-5-methylcoumarin derivative [3]: the aromatic system formed by two broad doublets at d 7.14 and 7.22, and a double doublet at 7.49; the CH3-9 at 2.69 ppm and the singlet H-3 at d 5.97. The coupling constants of the sugar moiety protons as well as the 13C NMR signals suggested the presence of a b-D-glucopyranose which was confirmed by 2D NMR means (Table 2). The long-range couplings of C-4 with the anomeric proton H-1’ and the singlet H-3 confirmed the position of the sugar ring. The complete 13C NMR shift assignments were done on the basis of 2D NMR experiments and in agreement with those previously described[16]. Coumarins have shown a broad pharmacological profile [17], including anticancer and antioxidant activities [18].So far some coumarines have shown cytotoxicity against A2780 ovarian cancer cells with IC50 values ranging between 3.2 and 11.4 mg/mL [19]. Here we measured the radical scavenging activity in the ABTS test as well as the antiproliferative effect against cell line CaCo-2 human colon carcinoma cells.
A preliminary antioxidant evaluation of the extract and pure compounds was done using the ABTS free radical scavenging test (Table 3). The EtOH extract showed an interesting activity for further assay-guided fractionation. Compound 1 seems to be one of the responsible metabolites for the activity showed by the EtOH extract, its activity could be due to the presence of phenolic parts in the coumestan derivative [20]. Compound (2) is less active but still interesting for further studies.
Table 3. Preliminary antioxidant evaluation using ABTS test
| Sample | Conc. | %I |
| EtOH extract | 2.7 mg/mL | 86% |
| Compound (1) | 2.7 mg/mL | 99% |
| Compound (2) | 2.7 mg/mL | 78% |
| Quercetine (patron) | 2.7 mg/mL | 100% |
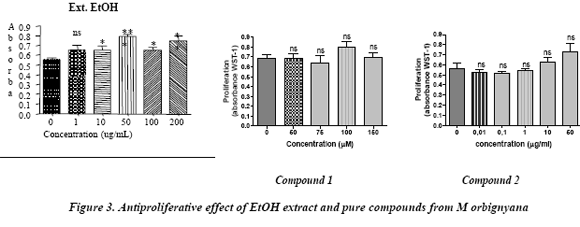
The cytotoxic evaluation did not reveal any activity on cancer colon cells. The EtOH extract did not show an antiproliferative effect on CaCo-2 cells between 1 to 200 mg/ml and the pure compounds showed no cytotoxic effect at > 0.1 µM
The cell proliferation rate on CaCo-2 cells was assayed using WST-1, which is a terazolium salt, metabolized to a red formazan. The formation of formazan is proportional to the mitochondrial dehydrogenase activity, which in turn correlates with the number of the viable cells. After the incubation of the cells with plant extracts the absorbance was read at 405 nm. If the absorbance is lower than 0.5 the inhibition is significant. The figure 3 shows that the EtOH extract (1-200 µg/mL) and the coumarines (0.01 – 150 µg/mL) did not show a significant effect, even when compound 2 shows an absorbance near to 0.5 between 0.01 and 0.1.
EXPERIMENTAL SECTION
General Experimental Procedures
The melting points (uncorrected) were recorded on a Sanyo Gallenkamp Melting Point Apparatus. Mass spectra (HREIMS) were measured in a Waters Micromass Q-TOF apparatus. 1H and 13C NMR spectra and two-dimensional experiments were recorded with a Bruker DRX400 using DMSO as solvent; chemical shifts are reported in d units (ppm) and coupling constants (J) in Hz. Sephadex LH-20 was used for gel filtration; Silica gel (E.M. Merck, 70-230 mesh) and silica gel G-60 (E.M. Merck) were used for CC and VLC, respectively, while aluminum plates impregnated with silica gel 60 F254 (E.M. Merck) were used for analytical (0.25 mm) TLC analyses. Spots on chromatograms were detected under UV light (254 and 365 nm) and by spraying the plates with 10% H2SO4, followed by heating.
Plant material
The aerial parts of Mutisia orbignyana Wedd. were collected from Alto Saucari (Oruro, Bolivia) in June of 2004 by researchers of the Natural Products Laboratory (IIQ/UMSA). The sample was identified by Lia de Michel, expert of the National Herbarium of Bolivia, where a voucher specimen is preserved.
Extraction and isolation
Dried leaves (1.5 kg) were powdered and extracted with ethyl alcohol (3.5 liters) for 72 h. The extract was concentrated in a rotaevaporator, and defatted with dichlorometane. The thick brown residue (35 g) 5 g was chromatographed on silica gel eluting with solvents of increasing polarity using dichlorometane-methanol-water. Compound 1 (15 mg) was obtained and purified further by recrystallization in MeOH. The fractions that contained compound 2 (600 mg) were rechromatographed on Sephadex LH-20 using methanol as eluent and then crystallized afforded 100 mg of pure compound.
Compound 1: Mutisifurocoumarin, pale yellow crystals, mp. 296-299 °C, HREIMS m/z 283.0595., calc. for C16H10O5+H+ 283.0601. 1H NMR (DMSO-d6, 400 MHz): d 7.47 dd (1H, dd, J 8.1, 7.6 Hz, H-7), 7.34 d (1H, d, J 8.1 Hz, H-8), 7.23 d (1H, d, J 7.6 Hz, H-6), 7.29 (1H, s, H-6’), 7.19 (1H, s, H-3’), 2.79 (3H, s, H-9). 13C NMR (DMSO-d6, 100 MHz): d 158.8 (C-2), 157.4 (C-4), 153.0 (C-8a), 149.4 (C-1’), 144.7 (C-4’), 126.7 (C-6), 114.8 (C-8), 113.4 (C-3), 111.8 (C-4a), 105.5 (C-2’), 104.7 (C-6’), 98.8 (C-3’), 20.8 (C-9).
Compound 2: 5-methylcoumarine-4-b-glucoside, white needles crystals, mp. 148-150°C, HREIMS m/z 339.1067., calc. for C16H19O8+H, 339.1074. 1H NMR (DMSO-d6, 400 MHz): d 7.49 dd (1H, dd, J 8.1, 7.6 Hz, H-7), 7.22 d (1H, d, J 8.1 Hz, H-8),7.14 d (1H, d, J 7.6 Hz, H-6), 5.97 (1H, s, H-3), 5.19 (1H, d, H-1’), 3.72 (1H, dd, J 9.9, 5.3 Hz, H-6’A), 3.50 (1H, dd, J 9.9, 5.3 Hz, H-6’B), 3.50 (1H, m, H-5’), 3.35 (1H, m, H-3’), 3.40 (1H, m, H-2’), 3.20 (1H, m, H-4’), 2.69 (3H, s, H-9). 13C NMR (DMSO-d6, 100 MHz): d166.6 (C-4), 161.3 (C-2), 154.3 (C-8a), 137.1 (C-5), 132.0 (C-7), 127.8 (C-6), 114.9 (C-8), 113.8 (C-4a), 99.9 (C-1’), 93.0 (C-3), 77.4 (C-5’), 76.6 (C-3’), 73.1 (C-2’), 69.5 (C-4’), 60.6 (C-6’), 23.2 (C-9).
Scavenging Activity of ABTS Radicals
The extract and the major isolated coumarin were tested by this screening method, which can evaluate the radical scavenging capacity of different substances including hydrophilic to lypophilics.
The radical monocationic (ABTS.+), previously formed from 2,2-azinobis-(3-ethylbenzotialzolina-6-sulfonic acid) by oxidation with ammonium per sulfate, gives a colored compound that is stable after 12-16 h [21]. The scavenging activity of the different samples were measured in function of the grade of discoloration caused by each one when catches an odd electron from monocationic radical ABTS×+ or sets a free proton. Samples to be proved were prepared in methanol, 1 mL of ABTS×+ was used with 10 µL of each sample solution, the reaction is stabilized in the next 4 min. All determinations were run in triplicate. The percentage inhibition of absorbance at 734 nm for each sample was calculated relative to a blank absorbance (methanol) and Quercetin l000 µM as positive control [22], [23]. The inhibition percentage (%I) is calculated by the following formula:
% inhibition= 100 [(X - Abs Ref(Ql000)/ (AbsABTS - AbsRef(Q1000))].
Cell growth inhibition studies
The ethanolic extract and the two isolated coumarines were evaluated to measure their cell growth-inhibiting potential in CaCo-2 cells. CaCo-2 human colon cancer cells were obtained from the European branch of American Tissue Culture Collection. Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal calf serum (FCS) were purchased from Gibco, and the WST-1 reagent was purchased from Roche.
CaCo-2 cells were cultured in DMEM with L-glutamine, containing 100 IU/ml penicillin, 10 µg/ml streptomycin and 10% (v/v) heat-inactivated FCS. Cells were maintained in 25 cm2 culture flasks at 37 ºC in a humidified incubator containing 95% air and 5% CO2. For assays of cell proliferation the cells were detached with 0.05% trypsin / 0.02% EDTA, resuspended to a concentration of 1 x 105/ml and seeded into 96-well plates (Falcon) for 24 h. After 24 h, the plant extracts dissolved in DMSO (1% final concentration) was mixed with 200 µl medium and added to each well, followed by incubation for 24 h. Controls were treated with the same amount of DMSO. Ursolic acid (40 µM) was used as a positive control.
The cell proliferation rate was assayed by use of WST-1 which is a tetrazolium salt, metabolized to a red formazan. The formation of formazan is proportional to the mitochondrial dehydrogenase activity, which in turn correlates with the number of the viable cells. After the incubation of the cells with plant extracts, 20 μl of WST-1 reagent was added to each well. The plate was incubated for 1 h at 37ºC and the absorbance was read at 405 nm using 655 nm as background. The extract was tested at the concentrations of 200µg/mL, 100µg/mL, 50µg/mL, 10µg/mL and 1µg/mL; the two coumarines were tested between 0,1µM and 1000µM.
ACKNOWLEDGEMENTS
The authors wish to thank to the Swedish Agency SIDA for financial support.
REFERENCES
[1] Rozema J; Bjorn LO; Gaberscik A; Hader DP; Trost T, Germ M; Klinsch M; Groniger A; Sinha RP; Lebert M; He YY; Buffoni-Hall R; de Bakker NVJ; van de Staaij J; Meijkamp BB. J. Photochem. Photobiol., B 2002:66, 2. [ Links ]
[2] Mollinedo P, Sacedo L, Vila JL, Ferreira M, Dajas F, Almanza GR. Rev. Bol.Quim. 2001; 18: 59. [ Links ]
[3] Zdero C, Bohlmann F, Solomon J. Phytochemistry 1988; 27: 891. [ Links ]
[4] Villegas LF, Fernández ID, Maldonado H, Torres R, Zavaleta A, Vaisberg AJ, Hammond GB. J. Ethnopharmacol. 1997; 55: 193. [ Links ]
[5] Giberti GC. J. Ethnopharmacol. 1983, 7, 321–341.
[6] Daily A, Seligmann O, Nonnenmacher G, Fessler B, Wong SM, Wagner H. Planta Medica 1988; 54: 270. [ Links ]
[7] Catalano S, Cioni PL, Flamini G, De Feo V, Morelli I, International Journal of Pharmacognosy 1995, 33: 73–74.
[8] Catalano S, Cioni PL, Panizi L, Morelli I, 1995. J. Ethnopharmacol. 1998; 59: 207.
[9] Viturro C, Molina A, Schmeda-Hirschmann G. Phytotherapy Research 1999; 13: 422. [ Links ]
[10] Pérez-García P, Marín E, Adzet T, Cañigueral S. Phytomedicine 2001; 8: 31. [ Links ]
[11] Juarez BE; Mendiondo ME. Pharmaceutical Biology 2003; 41: 291 [ Links ]
[12] Zdero C. Bohlmann F, King RM, Robinson H. Phytochemistry 1986; 25: 509 [ Links ]
[13] Bohlmann F, Zdero C, Ngo LV. Phytochemistry 1979; 18: 99 [ Links ]
[14] Bittner M, Silva M, Rozas Z, Papastergiou F, Jakupovic J. Phytochemistry 1994; 36: 695. [ Links ]
[15] Viturro CI, de la Fuente JR, Maier MS. J. Nat. Prod. 2004; 67: 778. [ Links ]
[16] Hernandez-Carlos B, Burgueno-Tapia E, Joseph-Nathan P. Magn. Reson. Chem. 2003; 41:962 [ Links ]
[17] Borges F, Roleira F, Milhazes N, Santana L, Uriarte E, Activity Current Medicinal Chemistry, 2005, 12: 887-916. [ Links ]
[18]Kulkarni MV, Kulkarni GM, Lin CH, Sun CM, Current Medicinal Chemistry, 2006, 13: 2795-2818. [ Links ]
[19] Prakash VS, Chaturvedula, Schilling JK, Kingston DGI, J. Nat. Prod. 2002, 65: 965-972 [ Links ]
[20] Hoult JRS, Paya M. Pharmacology Group, King’s College London, London. General Pharmacology. 1996, 27(4):,713-122.
[21] Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C, Free Radical Biology & Medicine 1999: 26(9/10): 1231 [ Links ]
[22] Cioffi G, D’Auria M, Braca A, Mendez J, Castillo A, Morelli I, De Simone F, and De Tomais N, J. Nat. Prod. 2002, 65: 1526-1529
[23] Pellegrini R, Proteggente A, Pannala A, Yang M, Rice Evans C, Biochem. Soc. Trans, 1998; 1231 [ Links ]