Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Boliviana de Química
On-line version ISSN 0250-5460
Rev. Bol. Quim vol.25 no.1 La Paz Dec. 2008
LIPOLYTIC ENZYME PRODUCTION BY HALOPHILIC/HALOTOLERANT MICROORGANISMS ISOLATED FROM LAGUNA VERDE, BOLIVIA
Mariana Niño de Guzmán;a,* Virginia A. Vargas;a Henry Antezana; a Michal Svobodab
aCentro de Biotecnología − UMSS, Cochabamba - Bolivia;
bLaboratoire de Chimie Biologique − Université Libre de Bruxelles, Belgium
Key Words: Halophile, halotolerant, lipolytic enzymes, lipases, Laguna Verde
ABSTRACTBacterial strains isolated from water samples from Laguna Verde, Potosi-Bolivia and deposited at Biotechnology Center, Cochabamba-Bolivia, were subjected to lipolytic enzyme production studies. Among these, 10 strains were selected as positive in lipolytic enzyme production in solid medium, using olive oil as sole carbon source. Selected strains were subjected to morphological and biochemical studies. Enzyme production in liquid medium identified strain LV01 as the best enzyme producer with 0.079 U/ml lipolytic activity. Partial purification by ion-exchange chromatography of the lipolytic enzyme, produced by strain LV01, presented specific enzyme activity of 0,138 U/mg of eluted protein with approximately 77 KDa size. Partial characterization of the produced enzyme revealed its display maximum activity values at pH 9, 30°C y 1.7M NaCl , thus exhibiting remarkably haloalkalitolerant properties. The 16S rDNA gene sequence analysis showed 99% similarity between strain LV01 and Bacillus pumilus (embAJ494727).
*Corresponding author: marianitaruf@hotmail.com
INTRODUCTION
Extremophilic microorganisms supply industries with a variety of biotechnology tools like outstanding stable enzymes which remain active in a wide range of pH, temperature and extremes saline concentrations. Extreme environments include conditions such as high salinity, acidity, alkalinity and extreme temperature, which are predominant conditions in a variety of industrial processes. Microbial population that can live and reproduced in this kind of environment are called extremophiles, and its microbiology its being widely studied around the world, since they produce enzymes able to work under such conditions and can be used in biotechnological and industrial potential applications [8, 18, 23].
Halotolerant and halophilic microorganisms grow in environments with high salinity concentrations. Halophilic bacteria are found in different environments such as salt lakes, saline soils and salted foods [14, 23]. The majority of halophilic microorganisms studied so far produce compounds with great potential in industrial processes and they have physiological properties which facilitate its use with commercial aims. Enzymes produced by halophilic microorganisms have developed particular features which confer them stability and solubility at high salt concentrations, thus, low water concentration. However, in spite of a growing interest in the use of halophilic enzymes for biotechnological applications, there are relatively few reports in the literature about their production and characterization [3].
Lipolytic enzymes (EC 3.1.1.x), represent a hydrolases group, which specifically work over carboxylic ester. Lipases are defined as a carboxylesterases which catalyses the hydrolysis and synthesis of long-chain acylglycerols with trioleoylglycerol being the standard substrate. Lipases are able to develop hydrolysis, esterification, perhydrolisis, alcoholysis, intersterification and aminolysis reactions. Lipases shape a versatile group of enzymes, due to a big amount of catalyzed reactions, therefore, a high potential of applications such as detergent, flavor development, paper recycle, chemical systems, racemic mixtures, and so on [9, 21]. A variety of microbiological origin lipases with different properties and substrates specificity has been isolated and characterized so far. Lipases and esterases have been recognized as very useful biocatalysts due to its wide-ranging versatility in industrial applications. Practical use of microbial lipases has developed a great interest concerning the improvement of both the producing strains and the biochemical properties of lipolytic enzymes [17].
Biotechnological potential of lipases, due to their estereospecificity, is enormous and they attracted a high interest for food, agricultural, chemical, pharmaceutical, medical and cosmetic industry among other areas. Most of catalyzed reactions by lipases show a high selectivity and efficiency, and they occur under middle conditions. These reactions occur without added cofactors and with low energy requirements, this properties contribute to reduce industrial conversions cost, and justify the growing interest in lipases. In the present work, we studied halophilic and halotolerant microorganisms isolated from Laguna Verde, Potosi-Bolivia, and its ability to produce lipolytic enzymes using an induction medium, moreover the best enzyme producer has been characterized and identified at species level.
RESULTS AND DISCUSSION
Screening of bacterial strains for extracellular lipolytic activity
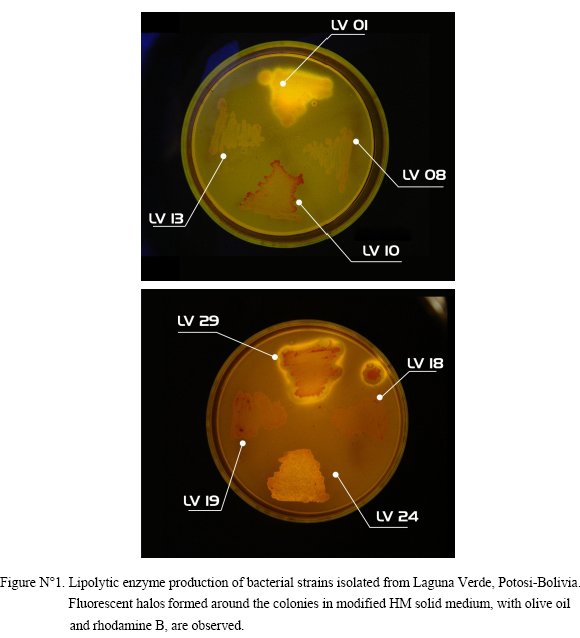
Halophilic and halotolerant bacterial strains isolated from Laguna Verde (Potosi - Bolivia), in total number of 34 were studied. Lipolytic activity of the isolates was detected in solid medium with olive oil as sole carbon source. Production of extracellular lipolytic enzymes was positive in 10 of the strains tested. This activity was observed through the formation of fluorescent halos visible under ultraviolet light to 350nm (Fig 1).
Bacterial lipases are mostly extracellular; where the biggest factor in the expression of lipolytic activity has always been the requirement of an adequate carbon source, in this case triacylglycerols. The use of fatty acids provides hydrolysable esters among other compounds that induce a lipolytic activity of microorganism. The lipases are produced through bacterial growth, where this production period can vary from a few hours to several days depending on the bacteria and environmental conditions [7].
Lipolytic activity
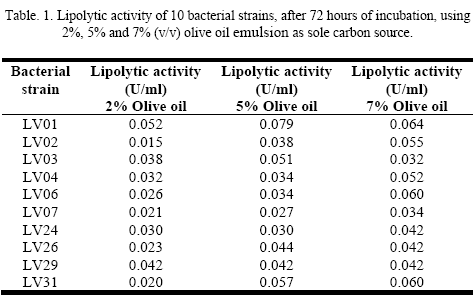
Lipase producing bacterial strains that tested positive in solid medium were grown in a minimal liquid medium, having as the sole carbon source olive oil emulsion, at concentrations of 2%, 5 % and 7% v/v. Lipolytic activity in the culture supernatant was detected through the free fatty acids increase concentration in the medium, as a result of a hydrolysis reaction carried out by lipolytic enzymes (Table 1.).
Strain LV01 registered the maximum lipolytic activity value, 0.079 U/ml, after 72 hours of incubation, at 30°C, 200 rpm and 5% (v/v) olive oil concentration. Enzyme activity values at 2% (v/v) olive oil concentration probe to be lower compare to those obtained at higher olive oil concentration. In a growing medium consisting of an emulsion, olive oil in this case, this concentration suggests that is not sufficient to achieve an optimal interface for lipolytic enzyme production. On the other hand, lower activity observed with higher substrate concentrations, 7% (v/v) olive oil, may be due to the inhibition by excess substrate.
Therefore, the bacterial strain LV01 was selected to be subjected to more detailed study related to enzyme production and phylogenetic analysis.
Morphological and physiological characteristics of the isolates
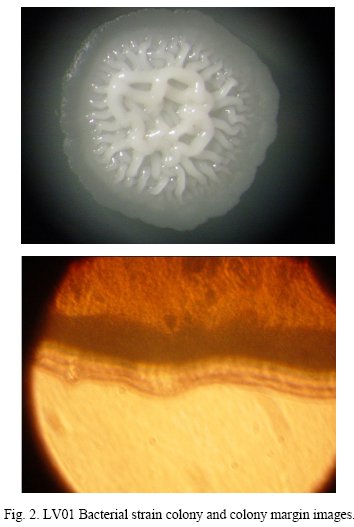
Bacterial strain LV01, identified as the best enzyme producer in liquid medium, is composed of Gram positive, short bacilli and mobile cells. The formed colonies are opaque white color and irregularly shaped, presenting a rough appearance at the center (Fig. 2).
Optimal growth occurs at 0% (w/v) NaCl concentration and can be classified as halotolerant, by the fact that it tolerates a maximum of 15% (w/v) NaCl concentration. Bacterial growth has been registered at a wide temperature range, between 4°C and a 45°C and pH values ranging from 5 up to 10.
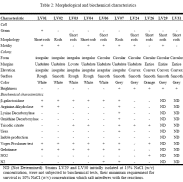
Morphological and biochemical characteristics, using the biochemist kit API 20E, of the isolates tested positive in enzyme production are summarized in table 2.
All the bacterial strains probed to be negative in the use of carbohydrates, not to consume glucose, mannitol, inositol, sorbitol rhamnosa, sucrose, melibiose, amygdaline and arabinose, and also in H2S production. However, they tested positive to tryptophan deaminase reaction. Even more, all of them were positive in ortho-nitrophenyl-βD-galactopiranosidase, Arginine dihidrolase, Tryptophan and Gelatinase deaminase production. Likewise, they were positive for acetoíne (Voges Proskauer) production. The isolates were tested negative in carbohydrates fermentation.
Several strains, genera and species of microorganisms present characteristic patterns in the use of different substrates, metabolic products and sugars fermentation. Specific characteristics selection for patterns development proves to be a tool for partial identification of a variety of species and is supported by a phenotypic analysis correlation. There is a variety of commercial equipment that can employ a battery of 20 or more biochemical tests in combination with phenotypic characteristics [10].
Phylogenetic analysis of strain LV01
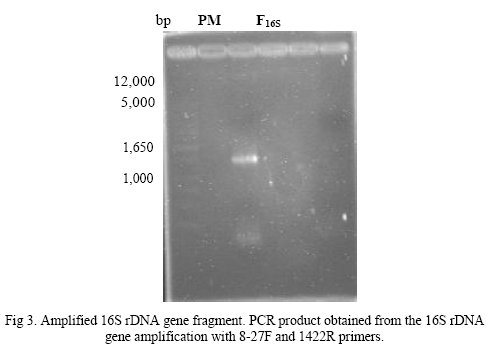
The 16S rDNA sequences obtained from a PCR amplification of the gene that encodes the ribosomal RNA using universal primers 8-27F and 1422R 16S (Fig. 3), were aligned to those available in the NCBI database for comparison with other sequences of the gene database of GenBank.
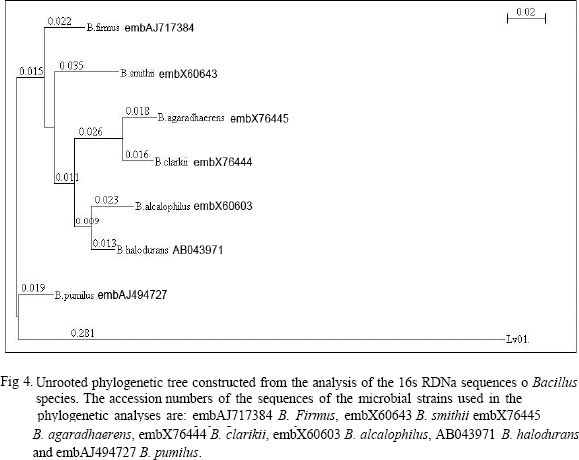
Isolate LV01 was associated with members of the diverse Bacillus spectrum showing sequences similarities between 95% and 98% with B. firmus, B. Smithii, B. Agaradhaerens, B. clarikii, B. alcalophilus, B. halodurans and revealing 99% sequence identity to Bacillus pumilus. The phylogenetic tree constructed from analysis of the 16S rDNA sequences of different species of the genus Bacillus, based on the similarities of the gene sequences, showed the position of the strain LV01 (Fig. 4). Strain LV01 will be identified as Bacillus pumilus LV01 in the subsequent studies.
Partial purification by ion exchange chromatography
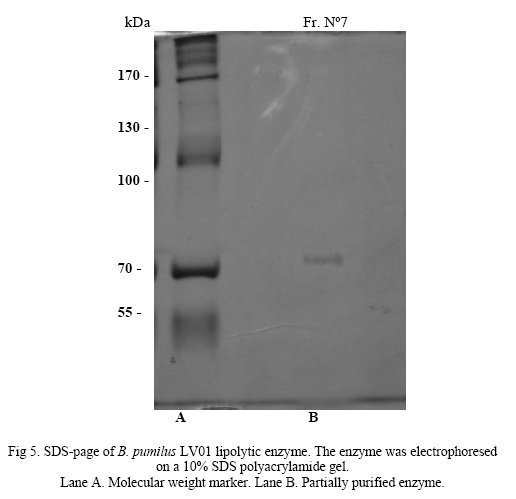
Lipolytic enzyme produced by B. pumilus LV01 was partially purified in a Macro-Prep DEA(BioRad) column equilibrated with 1M Tris/HCl buffer. The proteins were eluted with 1M Tris/HCl buffer and NaCl 100 mM. Higher values of enzyme activity were detected in fraction N° 7 and specific activity was 0,138 U/mg of eluted protein. This partial purified enzyme was electrophoresed on 10% (w/v) SDS-PAGE and a single band was observed. Using standard protein markers the size of the partially purified enzyme was found to be around 77 kDa (Fig. 5).
Molecular weight of this band, is significantly higher than lipolytic enzymes obtained from B. pumilus[5, 11]. However, we cannot exclude that minor unstained protein of lower molecular weight is actual lipase.
Characterization of partially purified lipolytic enzymee
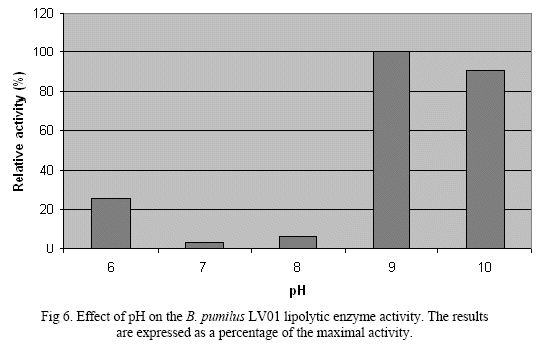
The partially purified enzyme was subjected to diferent incubation conditions. The effect of the pH on the activity was tested at diferent pH values. The enzyme presented activity over a broad pHrange, from 6 to 10, being more active at pH of 9 and inactivated at pH value of 5. Therefore, it can be characterized as an alkalophile enzyme (Fig. 6).
A comprehensive review of all bacterial lipase done by Gupta, et all. 2004, states that maximum activity of lipase at pH values higher than 7.00 has been observed in many cases. Bacterial lipases have a neutral or alkaline optimum pH with the exception of lipase from P. fluorescens SIK W1 that has an acid optimum pH of 4.8. But in general bacterial lipases are stable in a wide range of pH, from 4 to 11 pH.
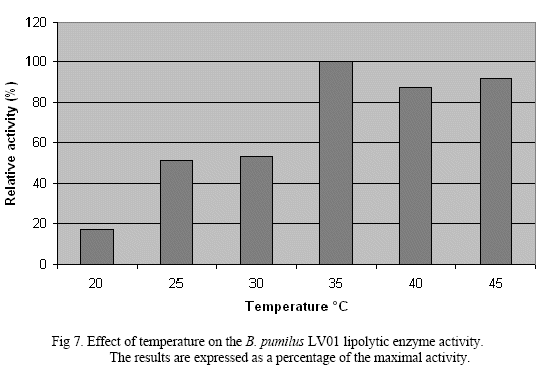
The temperature preference of this enzyme reveals higher activity values at temperature range of 35 - 45ºC. Also, this enzyme is not active at lower temperature ranges, probably due to low temperatures are not conducive to the kinetics ofenzymatic reaction (Fig. 7).
Bacterial lipases have benn reported with an optimum temperature within a range of 30 - 60°C. But there are some strains which even have a thermo-stability at upper temperatures up to 100ºC[7].
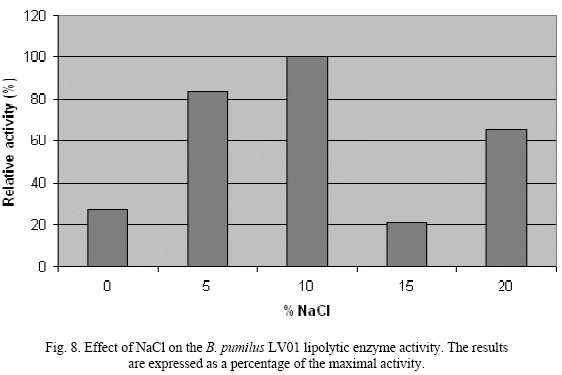
The lipolytic enzyme presents a wide range of tolerance to NaCl, becoming even more active at concentrations between 5% and 10% NaCl, showing a maximum activity at this last value concentration (Fig. 8).
This behavior might involve the fact that this is a salt dependent enzyme, either for stability or activity, especially since it is a lipolytic enzyme produced by a halotolerant strain, this behavior has been sugested previously by Ventos et al, 1998. The effect of different metallic salts in lipase activity was studied, and it became apparent that the CaCl2 stimulates enzyme activity, while other salts have an antagonist effect. But in general, the influence of different metal ions is specific and different for each type of bacterial lipase depending on the origin of the same [4].
EXPERIMENTAL
Screening for microorganisms with lipolytic activity
The bacterial strains studied are part of a Microorganisms Bank from the Biotechnology Center (FCyT-UMSS). Halophilic and halotolerant strains were isolated from water samples collected from Laguna Verde, Potosi - Bolivia. The lagoon presents a salinity variation between 49-55% [15]. The isolation was carried out using HM (Heterotrophic Microorganisms) culture medium (Commposition % ww/v: yeast extract 1.0; peptone, 0.5; glucosse, 0.1; KCl, 0.05; MgSO4.7H2O 0,0.025; CaCl2.2H2O, 0.009 ; NaBr, 0.006) at diferent salt concentrations (NaCl). A total number of 34 bacterial strains, halophilic and halotolerant, were selected from which 14 were isolated at 0% NaCl, 5 to 5% NaCl, 7 to 10% NaCl and 8 to 15% NaCl (w/v) [16, 19].
Plate assay for bacterial lipases
The halophilic and halotolerant bacteria were cultured at 30 °C in a modified HM solid medium, with 0.3% o f yeast extract, replacing the peptone and glucose and with olive oil as sole carbon source. Olive oil, in this case 3% v/v, is an ideal substrate for lipases in a hydrolysis reaction, and Rhodamine B acts an indicator of the reaction. The production of lipases withh hydrolytic activity was revealed by a fluorescent halo around the colonies [12].
Culture conditions for lipolytic enzyme production
Bacterial strains tested positive is solid medium were grown in erlenmeyers flasks containing 160 ml of HM modified medium with 2%%, 5% and 7%% of olive oil, and incubated in a shaker at 200 rpm, 30°C for 4 days. Microbial growth wa s observed every 12 hours by monitoring the increase in optical density at 680 nm and samples of 1 ml were collected in order to analyze enzyme activity [3, 22]. The bacterial strains selected from the previous tests were inoculated again in modified HM liquid medium, with the corresponding olive oil concentration and incubated in a shaker at 2200 rpm and 330ºC. Once the enzyme production reaches its peak the medium was centrifuged at 10000 rpm for 15 min. in order to precipitate all celular material. The culture supernatant, which constitutes the enzymatic crude extract, was recoveered for further analysis.
Activity assay
Lipolytic activity was assayed using the titrimetry method [2]. The free fatty acids were titrated adding 0,001 of a NaOH 0.001N solution in order to maintain the pH at a constant end point value. Phenolphthalein was used as an indicator, where one unit of lipase is defined as the amount of enzyme which liberates 1 mol of fatty acid/min. (1ml of 0.001N NaOH is equivalent to 1 mole of fatty acids liberated per minute) [4].
Morphological and physiological characterization
The bacterial strain that presented higher values of lipolytic activity in liquid medium was taxonomy characterized. Phenotypic characteristics were considered such as growth conditions, microscopic and macroscopic characteristics of the colony and cell, respectively, and biochemical characteristics, for the latter biochemical kit API 20E was used [16, 18, 19].
16S rDNA amplification and sequencing
Genomic DNA extraction was realized with the phenol-chloroform extraction protocol and agarose gel electrophoresis, to verify DNA quality. Genomic DNA extracted was subjected to an amplification by PCR (Polymerase Chain Reaction) of the gene that encodes the 16S ribosomal RNA using the kit Big Dye terminator ® v3.1 Cycle sequencing kit, and 2 games of universal primers; 16f27A one (FD2) 16f27C (FD1) and 16r1488 [24], and 8-27F and 1422R [19]. The PCR amplified DNA fragments were purified using QIAquick gel extraction columns and sequenced by both strands in an automatic sequencer, Applied Biosystems 3730XL. The sequencing was carried out at the Department of Biotechnology at Lund University-Sweden.
Phylogenetic analysis
The sequence was assembled using the "sequencher" program, edited with the BioEdit program and then were compared against the GeneBank database (http://www.ncbi.nlm.nih.gov/Genbank/index.html). BLASTN program was used to search sequences deposited in the GeneBank that share similarities with the corresponding microorganism in the study. Subsequently, the program ClustalX was used to align the sequences from the database and with the help of the TreeView program the phylogenetic tree was visualized.
Lipolytic enzyme purification
The obtained enzymatic crude extract was subjected to ion exchange electrophoresis in a column of 1.5x18 cm containing Macro-Prep DEA(BioRad) column support, obtaining fractions of 3 ml. The identified fractions were subjected to protein and lipolytic activity determination. The amount of protein was determined by measuring absorbance to 280 nm, and lipolytic activity by titrimetry.
Electrophoresis and protein determination
Fractions that showed increased lipolytic activity were run in polyacrylamide gel electrophoresis (SDS-Page), with the objective of complementing the partial characterization of the enzyme and thus to determine their molecular weight on [20].
Determination of the optimum pH, temperature and NaCl concentration for lipolytic activity
The enzyme solution previously obtained was used for partial characterization. Enzymatic crude extract was subjected to different pH values: 5, 6, 7, 8, 9 and 10, in order to determine the optimum pH for the enzyme activity at 30 ºC and 0% NaCl. The pH buffers selected were (50mM): NaH2PO4/Na2HPO4 for pH of 5, 6, 7 and 8, and glycine/NaOH for pH 9 and 10. The optimum temperature for lipase activity was determined by measuring the reaction rate at 4, 20, 25, 30, 35, 40 and 55°C, at optimum pH value and 0% NaCl w/v. The effect of NaCl concentration on the enzyme activity was determined at different salt concentrations: 0, 5, 10, 15 and 20% NaCl (w/v) at pH and temperature optimum.
CONCLUSIONS
Salt lakes are special environments due to their characteristics, thus their phylogenetic diversity of aerobic populations possesses unique characteristics. This study was carried out with the aim of identified native microorganisms as potential sources for lipolytic enzymes with diverse and novel properties. Growth medium with olive oil as sole carbon source is a wide used technique, complemented with chromogenic substrate that facilitates identification. Bacterial strains isolated from Laguna Verde in a number of 10 tested positive in the production
of lipolytic enzymes. Isolate LV01 identified was as halotolerant had higher lipase activity. Partially purified enzyme obtained by chromatography had specific activity of 0.138 Units/mg of proteins. SDS-PAGE showed unique band of 77kDa i.e. with higher molecular mass compare with those previously reported for lipase from the same species [5, 11]. The enzyme exhibited maximum activity at pH value of 9, 35ºC and 1.7M NaCl, probing to be a potential candidate for industrial applications. Haloalkalitolerant enzymes are ideal such as detergent, leather and fine chemicals industries[1, 6, 13].
ACKNOWLEDGEMENTS
The study was supported by the Swedish International Development Agency (SIDA/SAREC) in a collaborative project between Centro de Biotecnología - Universidad Mayor de San Simón (Bolivia) and Lund University (Sweden). Additional support was provided by Concejo Interuniversitario de la Comunidad Francesa de Bélgica (CIUF).
REFERENCES
- Antipov, A.; Morozkina, E.; Sorokin, D.; Golubeva, L.; Zvyagilskaya, R. and L’vov, N. Characterization of Molybdenum_Free Nitrate Reductase from Haloalkalophilic Bacterium Halomonas sp. Strain AGJ 1_3. Biochemistry (Moscow), 2004, 70, 799-803.
- Beissoin, F.; Tiss, A.; Rivière, C. and R. Verger. Mhetods for lipase detection and assay: a critical review. European Journal of Lipid Science and Technology 2000,102, 133-155.
- Bhatnagar, T.; Boutaiba, S.; Hacene, H.; Cayol, J.; Fardeau, M.; Ollivier, B.; And J. Baratti. Lipolytic activity from Halobacteria: Screening and hydrolase production. FEMS Microbiology Letters. 2005, 248, 133–140.
- Bora, L. and M.C. Kalita. Production and Optimization of Thermostable lipase from a Thermophilic Bacillus sp LBN 4. The Internet Journal of Microbiology. 2007, 4 Number 1.
- Eggert T.; van Pouderoyen G., Pencreac’h G., Douchet I., Verger R., Dijkstra B. and Jaeger K. Biochemical properties and three-dimensional structures of two extracellular lipolytic enzymes from Bacillus subtili. ELSEVIER: Colloids and Surfaces B: Biointerfaces 2002, 26, 37–46.
- Gupta, S.; Kuhad, R.; Bhushan2, B. and Hoondal, G. Improved xylanase production from a haloalkalophilic Staphylococcus sp. SG-13 using inexpensive agricultural residues. World Journal of Microbiology & Biotechnology. 2001, 17, 5-8.
- Gupta, R.; Gupta, N. and P. Rathi. Bacterial lipases: an overview of production, purification and biochemical properties. Microbiology and Biotechnology. 2004.
- Hashim, Suhaila O. “An alkaline active maltooligosaccharide forming β-amilase from Bacillus halodurans”. 2004. Doctoral Thesis. Department of Biotechnology. Center for Chemistry and Chemical Engineering, Lund University. Sweden.
- Jaeger, K.E.; Dijkstra, B and T. Reetz. Bacterial Biocatalysts: Molecular Biology, Three-Dimensional Structures, and Biotechnological Applications of Lipases. Annual Review of Microbiology 1999, 53, 315 -351.
- Joklik, W.;Willet, H.;Bernard, A. and Wilfert, C. 1997. Microbiología. 20a edición. Ed. Panamericana. Buenos Aires, Argentina. pp. 31-43.
- Kim, H.; Choi, H.; Kim, M.; Sohn, C. and Oh, T. Expression and characterization of Ca2+-independent lipase from Bacillus pumilus B26. Biochimica et Biophysica Acta. 2002, 1583, 205– 212.
- Kouker, G. and Jaeger K. Specific and Sensitive Plate Assay for Bacterial Lipases. Applied and Enviromental Microbiology. 1986, 53, 211–213.
- Kumar, G.; Joo, H.; Koo, Y.; Paik, S.; and Chang, C. Thermostable alkaline protease from a novel marine haloalkalophilic Bacillus clausii Isolate. World Journal of Microbiology & Biotechnology. 2004, 20, 351–357.
- Kushner, D. J. Life in high salt and solute concentrations: Halophilic bacteria. In D. J. Kushner (ed.): Microbial life in extreme environments. 1978, Vol 4 N°7, 318-358.
- Navarro G. & M. Maldonado. 2002. Geografía ecológica de Bolivia. Ed. Simón I. Patiño. Cochabamba, Bolivia. 510 -525.
- Niño de Guzmán Mariana. 2005. Caracterización de bacterias aeróbicas: halofilas y halotolerntes presentes en la Laguna Verde, Potosí. Tesis de grado par optar al diploma academico en licenciatura en Biología. 22
- Prim, N.; Sánchez, M.; Ruiz, C.; Pastor, J.; and P. Díaz. 2003. Use of methylumbeliferyl-derivative substrates for lipase activity characterization.
- Quillaguamán, J., Hashim, S., Bento, F., Mattiasson, B. & Hatti-Kaul, R. (2004a submitted) “Poly (βhydroxybutyrate) production by a moderate halophile, Halomonas boliviensis LC1 using starch hydrolysate as substrate”.
- Quillaguamán, J.; Hatti-Kaul, R., Mattiasson, B., Alvarez, M. T. &. Delgado, O.,. “Halomonas boliviensis sp. Nov., an alkalitolerant, moderate halophile bacterium isolated from soil around a Bolivian hypersaline lake”. Int. J. Syst. Evol. Microbiol, 2004b, 57, 721-725.
- Ruiz, C., Blanco, A., Pastor and J., Diaz, P. Analysis of Bacillus megaterium lipolytic system and cloning of LipA, a novel subfamily I.4 bacterial lipase. FEMS Microbiology Letters 2002, 217, 263-267.
- Serrano, Alicia G. 2001. Estudio de la producción heteróloga de una lipasas del hongo Rhizopus oryzae en la levadura metilotrófica Pichia pastoris. Tesis doctoral.
- Vargas, V.; Delgado, O.; Hatti-Kaul, R. and Mattiasson, B. Lipase-producing microorganisms from a Kenyan alkaline soda lake. Biotechnology Letters 2003, 26, 81-86.
- Ventosa, A, Nieto, J. J. & Oren, A. Biology of Moderately Halophilic aerobic Bacteria. Microbiol Mol Biol Rev, 1998, 62, 504-544.
- Weisburg, W.G., Barns, S.M., Pelletier, D.A.. & Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriology. 1991, Vol. 173, No. 2, 697-703.