Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Boliviana de Química
On-line version ISSN 0250-5460
Rev. Bol. Quim vol.25 no.1 La Paz Dec. 2008
ELECTROPHORETIC STUDIES ON MEDICINALLY IMPORTANT
BINARY METAL COMPLEXES (SYSTEM: COPPER(II) / MANGANESE(II) / URANYL(II) – SARCOSINE)
Brij B. Tewari
Department of Chemistry, Faculty of Natural Science, University of Guyana, P. O. Box: 101110, Georgetown, Guyana.
Key Words: Electrophoretic technique, copper(II) complex, manganese(II) complex, uranyl (II) complex, stability constant, sarcosine
ABSTRACT
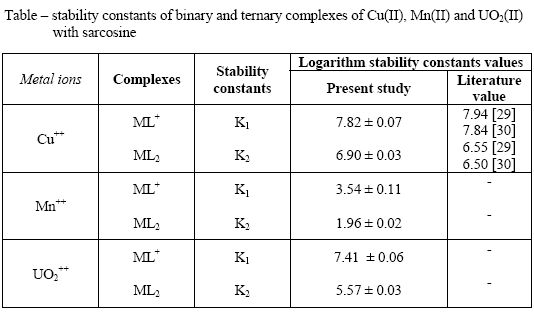
Quantitative indication of the process of forming a complex comes from the evaluation of the stability constants which characterize the equilibria corresponding to the successive addition of ligands. A technique involving the use of paper electrophoresis is described for the study of binary complex system in solution. The first and second stability constants of metal(II) – sarcosine complexes were found to be (7.82 ± 0.07, 6.90 ± 0.03); (3.54 ± 0.11, 1.96 ± 0.02) and (7.41 ± 0.06, 5.57 ± 0.03) (log stability constant values) for Cu(II), Mn(II) and UO2(II) complexes, respectively.
Corresponding author: brijtew@yahoo.com
INTRODUCTION
When the term stability is applied to coordination compounds (metal complexes) there can be two interpretations, thermodynamic or kinetic stability. Thermodynamic stability for metal complexes refers to the change in energy on going from reactance to products, i.e. ΔG for the reaction. Recall that:
Δ G = Δ H – T Δ S = - RT lnK
Where ΔH is enthalpy, ΔS the entropy and K is the equilibrium constant for the reaction. Kinetic stability refers to reactivity, generally ligand substitution. Substitution occurs extremely rapidly in some cases and extremely slowly in others. Complexes of former type are referred to as labile and those of latter type inert.
Paper electrophoresis has been applied to the study of metal complexes in solution and attempts have been made to determine the stability constants of complex species. A significant development on the determination of stability constants of biologically important complexes was made by Jokl[1]. The electrophoretic technique usually suffers from number of defects e.g. temperature rise during electrophoresis, adsorption and molecular sizing affect the mobility of charged moieties [2, 3]. The technique described here is almost free from these vitiating factors.
Sarcosine is the N-methyl derivative of glycine. It is a natural amino acid found in muscles and other body tissues. It is normally not detected in human blood or urine. Sarcosine has number of significance in biological systems [4-9]. Metal ions play an important role in biological systems. Copper and manganese classified as essential metals without which life could not sustain. Uranium is a toxic metal and harmful even at very low concentration. The normal recommended amount of copper, manganese, uranium elements in per day human diet is (0.5 – 6.0 mg), (0.4 – 10.0 mg) and (0.001 – 0.002 mg), respectively [10]. Copper, manganese and uranyl are well known for some biomedical applications and toxicity [11-19]. Communications [20-23] from our laboratory a new method for the study of metal complexes. The present method is the extension of the technique and reports my observation on copper(II) / manganese(II) / uranyl(II) – sarcosine system.
RESULTS
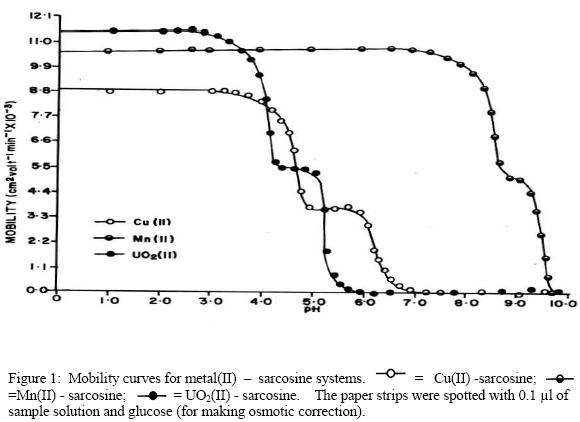
Chemical literature[24] confirms that anionic species of amino acids are the sole ligating species for metal ions. Hence a metal ion spot on the paper strip show a variation in composition of different ionic species of the amino acids in background electrolyte. So the mobility of the metal ion spot would depend upon the pH of the background electrolyte. The electrophoretic mobility of metal spot against pH gives a curve with number of plateaus shown in Figure. There are three plateaus in the curves of Cu(II), Mn(II) and UO2(II) cations. In this pH range sarcosine is present as a non-complexing species. Every plateau indicates the formation of certain complex species. A plateau is an indicating of pH range, where speed is practically constant. Figure reveals that second plateau lies in positive region indicating the cationic nature of 1:1 metal complex. With increase of pH mobility decreases giving rise to third plateau in zero region of mobility curve indicating formation of 1:2 metal complexes of neutral nature. Further increase in pH has no effect on the mobility of metal ions. Chemical literature also assigns prominent chelating properties of the unprotonated anionic species of sarcosine ruling out any such property to zwitterions [25]. In view of above observation the complexation of metal ions with sarcosine anion may be represented as:
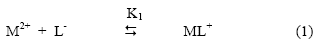
![]()
Where M2+ is Cu2+, Mn2+ and UO22+ cations; [L-] is sarcosine anion; K1 and K2 are the first and second stability constants, respectively. The two region of decreasing mobility represents conditions where two forms co-exist. The metal spot on the paper is thus a conglomeration of uncomplexed metal ions, 1:1 complex and 1:2 complex. The spot moving under the influence of electric field, the overall mobility is given by the well known equation of Jokl [26].
![]()
Where ux-p is the speed and fx-p is the mole fraction of the general complex [M( HpL)X] present in the conglomeration. Equn. [3] is transformed to equn. (4) on taking into consideration the different equilibria.
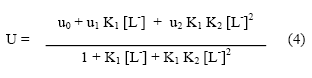
where u0, u1 and u2 are the mobilities of uncomplexed metal ions, 1:1 metal complex and 1:2 metal complexes, respectively.
Equn. (4) has been used for calculating the stability constants of the complexes of metal ions with sarcosine. For calculating the first stability constant, K1, the region between first and second plateau is pertinent. The overall mobility ‘U’ will be equal to the arithmetic mean of mobility of uncomplexed metal ions u0 and that of first complex u1 and a pH where K1 = 1/[L-].
The dissociation of pure sarcosine may be represented as:
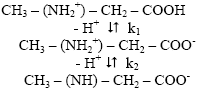
With the help of the dissociation constant of sarcosine [k1=102.20, k2=109.99 (paper electrophoretically obtained value)] the concentration of sarcosine anion [L-] is determined for the pH, from which K1 can be calculated. The concentration of chelating sarcosine species [L-] is calculated with the help of equation.
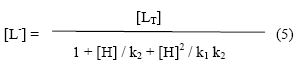
Where [LT] is the total concentration of pure sarcosine (0.01 M), k1 and k2 are the first and second dissociation constants of sarcosine, respectively.
The stability constant, K2, of the second complex can be calculated by taking into consideration the region between the second and third plateau of the mobility curve. Their calculated values are given in the table.
DISCUSSIONS
It is observed from the data in the table that the order of stability constants viz,
copper(II) > uranyl(II) > manganese(II)
are same for 1:1 and 1:2 binary complexes. This order of stability constant values find support from the work of Irving and Williams [27]. The stability of binary complexes follow the order
log K1 > log K2
The stability constant values are lower for (1:2) complexes. It is therefore, inferred that the corresponding tendency of a ligand decreases with a higher state of aggregation [28]. The maximum stability constant values of Cu(II) – sarcosine complex indicate strong bonding between copper cation and sarcosine anion. While minimum stability constant value of Mn(II) – sarcosine complex indicate weak bonding between manganese cation and sarcosine anion.
It is clear from the table that the calculated stability constant (K1 and K2) are approximately similar to literature values. The slight deviation in the values obtained from different sources is mainly due to the difference in temperature and ionic strength used by different workers. Through certain modification in experimental condition, we have tried to remove the errors, even then the precision of the method is limited to that of paper electrophoresis and the range of uncertainty in the result is ± 5%. The stability constants of metal complexes can be very easily calculated by this technique. The present electrophoretic technique have significant advantages over other methods reported in chemical literature for the determination of stability constants of metal complexes.
It may be concluded from these studies that Cu(II), Mn(II) and UO2(II) are significant for biological systems but as such they are toxic above the normal concentration level. The sarcosine can be used to reduce the level of these metal ions in the living cells. The modified simple electrophoretic technique has thus proved to be helpful in deciding whether a complex system is formed or not and if it is formed its stability constants can also be determined. The medicinally significant binary metal complexes of interest can be prepared on large scale at a fixed particular pH of the ligand (sarcosine) solution.
EXPERIMENTAL SECTION
Instruments
Systronics (Naroda, India) paper electrophoresis equipment horizontalcum-vertical type, model 604, has been used. The apparatus consisted of a PVC moulded double tank vessel. In our laboratory significant change in the instrument has been made. Two hollow rectangular plates covered with think polythene sheets have been used through which thermostated water is run for controlling the temperature. The tanks were closed with a transparent PVC moulded lid. The whole assembly is tight, which prevent moisture changes, which may upset the equilibria in a paper strip. This assembly design thus keeps to a minimum the disturbing effects of evaporation from the unwanted liquid flow in the paper. Each electrolyte tank contains a separate electrode chamber. The auxiliary unit is specially designed to operate either voltage mode or on current mode.
Elico (Hyderbad, India) model L1-10 having glass and calomel electrode assembly and working on 220 Volts/50 Hz established a. c. mains, were employed for pH measurements.
Chemicals
Metal perchlorate solutions were prepared by the precipitation of metal carbonates from their nitrates with sodium carbonate, which were washed with boiling water and treated with calculated amounts of 1% perchloric acid. There were heated and filtered. The metal contents of the filtrates were determined and final concentration was kept at 0.005 M. A 0.1% (w/v) solution of 1 - (2 – pyridylazo) – 2 – naphthol (PAN) (Merck, Darmstadt, Germany) in etanol was used for detecting the metal ions. 0.005 M glucose (BDH, AnalaR) solutions was prepared in water and used as an electro-osmotic indicator for the correction due to electro-osmosis. A saturated aqueous solution (0.9 ml) of silver nitrate was diluted with acetone to 20 ml. Glucose was detected by spraying with this silver nitrate solution and then with 2% ethanolic sodium hydroxide, when a black spot was formed.
Background electrolyte (BGE)
Stock solutions of 5.0 M perchloric acid (SDS, AnalaR grade), 2.0 M sodium hydroxide (AnalaR grade), 0.5 M sarcosine (BDH) were prepared. Each solution was standardized using the appropriate method. The BGE used in the study of binary complexes were 0.1 M perchloric acid and 0.01 M sarcosine. The pH of the solution was maintained by the addition of sodium hydroxide.
Procedure
Whatman No. 1 filter paper for chromatography was used for the purpose of ionophoresis. For recording observation of particular metal ion, two strips were spotted with the metal ion solution along with additional two spotted with glucose using 1.0 µl pipette and then mounted on the insulated plate. Each of the two electrolyte vessel was filled with 150 ml of background electrolyte containing 0.1 M perchloric acid and 0.01 M sarcosine. The paper become moistened with the background electrolyte solutions due to diffusion. The second insulated plate was placed on paper strips and then thermostated water (35°C) was circulated in the plates to keep the temperature constant. The lid was then placed on the instrument to make it air tight. It was left for 10 minutes to insure wetting of strips. Subsequently a direct 200 volts potential was applied between the electrodes for 60 minutes after which these strips were removed from the tank and dried. The metal ion and glucose spots were detected by specific reagents. The leading and tailing edge were measured from the marked centre point and the mean were taken. The distance moved by glucose was subtracted (in case of migration toward anode) to obtain correct path length. Migration towards anode and cathode were designated by negative and positive signs respectively.
Ionophoretic observations on metal ions recorded at various pH values of the background electrolyte obtained by adding NaOH solution. The ionic strength being maintained at 0.1 M. The observed mobility of migrant was calculated by using the formula.

after applying the correction factor the observed mobility is given as:
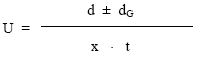
where U = mobility of metal ion / complex ion; d = mean of duplicate distances travelled by metal ion/complex ion; dG = mean of duplicate distance travelled by glucose spot; x = field strength; t = time for ionophoresis.
The dissociation constants of pure sarcosine were determined by the same paper electrophoresis technique. The two paper strips were spotted with pure penicillamine along with two with glucose using 0.1 M perchloric acid only in a background electrolyte. The ionophoresis was carried for 60 minutes as for metal ions. The ionophoretic speed was calculated. The speeds of the metal ions / complex ions are reported with pH values. The individual speeds of the duplicate spots were found to be fairly equal. A plot of mobility against pH is shown in Figure 1.
REFERENCES [ Links ]