Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Boliviana de Química
On-line version ISSN 0250-5460
Rev. Bol. Quim vol.23 no.1 La Paz 2006
ARTÍCULO ORIGINAL
LIFE ORIGINATES ON PRIMITIVE EARTH OR ON OTHER PLANETS IS STILL A SUBJECT OF CONTROVERSY
Brij Bhushan Tewari* & Rawl Wayne Webster
Department of Chemistry, Faculty of Natural Sciences, University of Guyana, P. O. Box: 101110, Georgetown, Guyana.
*Corresponding author: brijtew@yahoo.com
ABSTRACT
The bioorganic compounds found in the primitive terrestrial atmosphere or extraterrestrial environment like comets has been controversial. According to the first view it is well known that bioorganic compounds such as amino acids are easily formed in a mixture of methane, ammonia and water, that is a strongly reduced atmosphere, when spark discharges, ultraviolet light and X-ray irradiation are used as energy sources. According to the second view the primitive earth atmosphere was mildly reduced; therefore formation of amino acids and other bioorganic compounds were difficult to form. It is suggested that various kind of organic compounds exist in space, e.g. comets and meteorites.
INTRODUCTION
When comets and meteorites fall to planets, it is possible that they can supply organic compounds to planets. It is supposed that organic compounds in comets were formed in their precursor bodies, interstellar dust particles (ISDs). ISDs were covered with ice molecules. It was estimated that they contain water, carbon monoxide, methane and ammonia. According to third view which is midway between first and second view, the recent experiment indicates that amino acids are easily produced even in slightly reduced atmosphere by irradiating with highly energy particles. Cosmic rays might be an effective energy source of a biotic formation of amino acids and other bioorganic compounds in the primitive aspect of the earth as well as other planetary / cometary atmosphere. During the course of the chemical evolution, cyanide ions were found in abundance, cyanide is a strong ligand and formed a variety of complexes with metal ions. Metal ferrocyanides, mostly insoluble in water, could have played an important role as adsorbents, ion-exchangers and photosensitizers. Aluminum, stannous and vanadium ferrocyanides were synthesized and characterized by elemental and spectral studies. Adsorption of egg albumin (EA) and bovine serum albumin (BSA) on above synthesized metal ferrocyanides were studied in pH range 1.0 - 10.0. The adsorption of EA and BSA on metal ferrocyanides were studied by measuring concentration of albumins before and after adsorption at their corresponding λmax. Stannous and vanadium ferrocyanides were found to have maximum and minimum adsorption capacity for both adsorbates. The EA was found to have highest adsorption with all three adsorbents. Results of present studies supported the view of terrestrial origin of life. The term ‘chemical evolution’ has come to mean the chemical events that took place on the primitive, prebiotic earth (about 4.5 - 3.5 billon years ago) leading to appearance of the first living cell [1]. How life was created on the earth is a question that has long occupied theologian and philosophers. While Darwinian evolution has replaced spontaneous generation as a generally accepted explanation of the origin of species, science has not yet satisfactorily answered the question of how life arose on the planet. The realization of how old and large the universe is relative to our own solar system has led to speculation that there are other worlds that support life. Indeed, it has been suggested that life was seeded here from some other part of the galaxy. Earth supplied the nutrients [2]. An array of experimental findings suggest that the simple molecules required for the origin of life may have been available on the primitive earth. The well-known Miller - Urey electric discharge experiment yield HCN, amino acids and carboxylic acids [3]. Carbonaceous meteorites contain over 70 amino acids, some heterocyclic compounds, including purines and carboxylic acids. Other organics may have been synthesized under the reducing conditions present in hydrothermal system [4]. Although some of these organics may have been present on the primitive earth, there is no agreement on which was the most important for their formation. The Miller-Urey experiment requires the presence of either hydrogen gas or reduced carbon and hydrogen compounds, such as methane, carbon monoxide, and ammonia in the atmosphere of the primitive earth. Most geochemists believe that the earth’s crust was not reducing when life originated, so the volcanic emissions that resulted in the primitive atmosphere were also not reducing. The notion that comets, meteorites and asteroids delivered reduced organics to the surface of the primitive earth is not favored by both who believe that, when these bodies hit the earth’s atmosphere, the organic compounds present on them would have been hydrolyzed [5]. In the earlier studies of chemical evolution, the following scheme was widely accepted: (i) Active compounds such as hydrogen cyanide and formaldehyde were abiotically produced from strongly reduced terrestrial atmosphere by thundering, solar UV radiation, and so on. (ii) Reactions among these active compounds gave bioorganic monomers like amino acids, nucleic acid bases and sugars. (iii) These monomers polymerized together to give polypeptides and polynucleotides. (iv) Finally “life” was generated, based on these biologically important polymers. Recent findings and theories on the history of earth and other planets gave new insights to the chemical evolution and the origin of life. For example, the discovery of submarine hydrothermal vents in the late 1970’s [6] has drastically changed the image of the primordial sea where life was believed to be born. Whether the bioorganic compounds were formed in the primitive terrestrial atmosphere or in the extraterrestrial environment like comets, has been controversial. Miller [7] reported that bioorganic monomers such as amino acids were easily formed in a mixture of methane, ammonia, hydrogen and water (“a strongly reduced gas mixture”) by spark discharges. Urey [8] postulated that the primitive earth atmosphere was strongly reduced. Other energy sources such as ultraviolet light [9] and heat were also applied to the same kind of gas mixtures and amino acids were successfully found in the product. It is known that the primitive atmosphere of the earth was composed of carbon dioxide, carbon monoxide, nitrogen and water [9], that is, the slightly reduced gases, and that carbon monoxide is a major carbon compound found in extraterrestrial environments like comets [10]. It is not easy to form amino acids from these slightly reduced gases by electric discharge [11] or ultraviolet lights [12]. It is known that a wide variety of organic compounds are present in extraterrestrial environments. For example, infrared spectrometers on board Voyager 1 and 2 prevailed the presence of organic compounds in such solar system bodies as Jupiter and Titan (a Moon of Saturn) [13]. Among these extraterrestrial organic compounds, cometary organics are the most important since they could be the sources for the first terrestrial biosphere after their collisions onto the primitive Earth [14]. Mass spectrometers on board Vega 1 and Giotto showed that cometary dust contained a wide variety of complex organic compounds [15]. It has been proposed as hypothesis that these cometary organics were formed in ice mantles of interstellar dust particles (ISD) in dark clouds by cosmic rays and UV light. Kasamatsu et al [16] showed that icy mixture of carbon monoxide, ammonia and water (“simulated ISD ice mantles”) gave amino acid precursors. The third view which is midway between terrestrial and extraterrestrial origin of life indicates that amino acids are easily produced even in slightly reduced gases by irradiating with high energy charged particles. Cosmic rays might be an effective energy source for abiotic formation of amino acids and other bioorganic compounds in primitive atmospheres of the earth as well as other planetary / cometary atmosphere [17]. The present paper describes the interaction of simple egg albumin proteins (EA), bovine serum albumin (BSA), with aluminum, stannous and vanadium ferrocyanides.
RESULTS AND DISCUSSION
The relative percentage adsorption of the egg albumin and bovine serum albumin with metal ferrocyanides:
The calculated mass and relative percentage adsorption of the egg albumin and bovine serum albumin on the metal ferrocyanides is shown in Table 1 & 2, respectively.
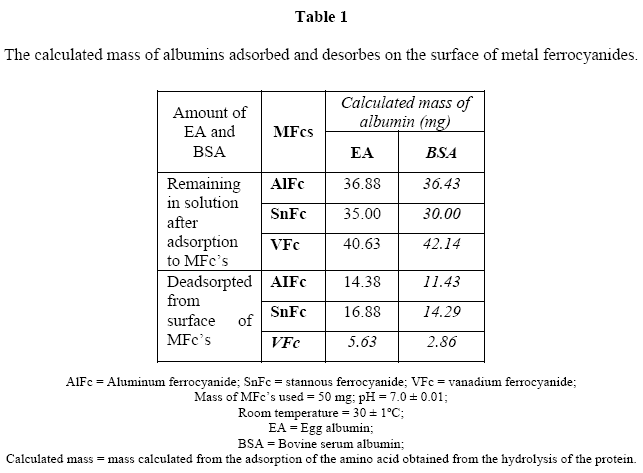
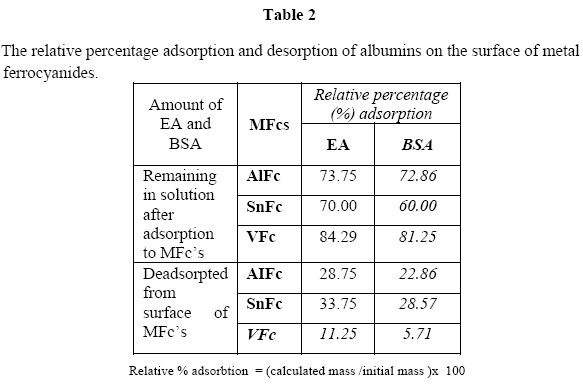
The results of the present studies showed that egg albumin in general adsorbed better to the metal ferrocyanide in comparison to bovine serum albumin. The relative percentage adsorption of egg albumin and bovine serum albumin on metal ferrocyanides follow the order:
Stannous ferrocyanide > aluminum ferrocyanides > vanadium ferrocyanide.
The relative percentage adsorption of both simple proteins viz. egg albumin and bovine serum albumin were found to be maximum for stannous ferrocyanide and the lowest for vanadium ferrocyanide.
Bovine serum albumin adsorbed on stannous ferrocyanide has the highest adsorption while egg albumin adsorbed to vanadium ferrocyanide had the lowest adsorption. In general, egg albumin had the highest adsorption while bovine serium albumin had the lowest.
The relative percentage description of egg albumin and bovine serum albumin with metal ferrocyanides.
The results of the present studies also showed that relative percentage desorption of egg albumin was found to be greater than bovine serum albumin with all three metal ferrocyanides studied. The relative percentage desorption of egg albumin and bovine serum albumin from the surface of metal ferrocyanides follow the order:
Stannous ferrocyanide > aluminum ferrocyanide. > vanadium ferrocyanide
The relative percentage desorption of both albumins were found to be maximum with stannous ferrocyanide and minimum with vanadium ferrocyanide.
Egg albumin adsorbed on the surface of stannous ferrocyanide and bovine serum albumin adsorbed on the surface of vanadium ferrocyanide were found to have maximum and minimum desorption properties, respectively. Present studies support the hypothesis of terrestrial origin of life.
EXPERIMENTAL
Chemicals
Potassium ferrocyanide [K4Fe(CN)6·3H2O)], aluminum chloride (AlCl3·6H20), stannous chloride (SnCl2·2H2O) and sodium vanadate (NaVO3) were obtained from BDH, Poole, UK. Egg albumin and bovine serum albumin were obtained from Sigma-Aldrich, USA. All chemicals used were of AnalaR grade and used without further purification. Solutions were prepared in doubly distilled water.
Synthesis of metal ferrocyanides
Aluminum ferrocyanide was prepared by Kourim’s method [18], whereas stannous ferrocyanide was prepared according to the procedure reported by Tewari et al. [19]. Aluminum ferrocyanide was prepared by adding slowly, a potassium ferrocyanide (167 ml; 0.1 M) solution to a solution of aluminum(III) chloride (50 ml; 0.1 M) with constant stirring. Reaction mixture was heated into a water bath for 2-3 h and then cured for 24 h. The precipitate was filtered, washed with distilled water and dried in an oven at 60ºC. A 0.25 M solution of potassium ferrocyanide and stannous chloride was mixed in the ratio (2:1) for the preparation of stannous ferrocyanide. The precipitate was cured at room temperature for 24 h, washed and dried at 40ºC. Vanadium hexacyanoferrate(II) complex was isolated by adding (10 ml: 1.0 M) HCl to mixture containing sodium vanadate (500 ml; 0.3 M) and potassium ferrocyanide (500 ml; 0.1 M) solution with constant stirring. Reaction mixture was heated on a boiling water bath for 3 to 4 h and then allowed to cool at room temperature overnight. The precipitate formed was filtered and dried at 50ºC. The dried products were ground and sieved to 125 µm mesh size.
Characterization of metal ferrocyanides
Aluminum, stannous and vanadium ferrocyanides are found to have light blue, dark blue and green colour, respectively. These complexes are amorphous solid and show no X-ray pattern. All three metal ferrocyanides were found to be stable at room temperature (30 ± 1ºC) and somewhat less stable on applied heat. All three metal ferrocyanides were also found to be stable in acids (HCl, HNO3, H2SO4) and bases (NaOH, KOH, NH4OH) solutions in the concentration range 0.1 – 2.0 M. Aluminum, stannous and vanadium ferrocyanides were characterized on the basis of elemental analysis and spectral studies. Aluminum, stannous, vanadium and iron were estimated by atomic absorption spectrophotometry on IL – 751 spectrophotometer. Carbon, hydrogen and nitrogen analysis was performed on CEST – 118, CHN analyzer. The analytical data for all three compounds are as follows: aluminum compound [found (1%) C – 13.20, H – 4.90, N – 16.60, Al – 9.90, Fe – 11.10. C6H22N6O11Al2Fe1. Yield – 93.35%], stannous compound [found (1%) C – 14.25, H – 1.75, N – 18.10, Sn – 38.75, Fe z– 10.90. C6H6N6O3Sn2Fe1. Yield – 93.55%], vanadium compound [found (%) C – 18.10, H – 2.00, N – 19.73, V – 24.10, Fe – 13.90. C6H8N6O4V2Fe1. Yield – 94.58%]. All three metal ferrocyanides show a broad peak at around 3650 cm-1, characteristics of water molecule and OH group. Also, a peak at around 1600 cm-1 due to HOH bending appeared in all the metal ferrocyanides studied. Two sharp bands at 2100 cm-1 and 600 cm-1 are characteristics of cyanide and Fe – C stretching respectively. Another sharp band at around 500 cm-1 probable show the presence of metal – nitrogen band due to polymerization.
Adsorption of egg albumin and bovine serum albumin on metal ferrocyanides.
In triplicate, 50 mg of egg albumin along with 50 mg of metal ferrocyanides and 10 ml of universal pH buffer was placed into the test tubes, it was then shaken for 4 h and then left to stand for an additional 4 h. The solution were then centrifused, the supernatant was then removed using a pipette and both supernatant and suspension were kept. The supernatant was hydrolyzed by adding 10 ml of 6N HCl with 1% phenol and lift to stand for 4 h, its absorbance reading was taken at their λmax and percentage protein remaining in the solution was determined. The same procedure was repeated for bovine serum albumin.
Desorption of protein from the surface of the metal ferrocyanides
The test tube containing the suspension was washed with 0.5 ml of buffer-salt mixture 0.5 M pipes or taps buffer and 0.2 M sodium Chloride (NaCl). The test tube walls were rinsed so as not to disturb and the mixture was immediately centrifuged. The supernatant was then removed and discarded. Then 1.0 ml of the buffer-salt solution was added to the suspension, the mixture was then agitated for 1 minute and allowed to stand for 1 hour until all the ferrocyanide settles. The supernatant was then removed with a pipetted and placed into a separate test tube [20]. Then 10 ml of 6N hydrochloric acid with 1% phenol was added to the removed supernatant, it was then left to stand for 4 hours, after which it absorbance readings were taken at the λmax as previously determined [21]. The procedure was then repeated for bovine serum albumin.
REFERENCES
1. LEMMON, R. M., Chem. Review, 1970, 70, 95. [ Links ]
2. BANIN, A. and NAVROT, J., Science, 1975, 189, 550.
3. FERRIS, J. P., Prebiotic Synthesis – Problems and Challenges: Cold Spring Harbor – Symposia on Quantitative Biology, 1987, 52, 29.
4. FERRIS, J. P., Origin of Life Evol. Biosphere, 1992, 22, 109. [ Links ]
5. FERRIS J. P., Biol. Bull., 1996, 196, 311. [ Links ]
6. SCHLESINGER G. and MILLER, S. L., J. Mol. Evol. 1983, 19, 376.
7. MILLER, S. L., Science, 1953, 117, 528. [ Links ]
8. UREY, H. C., The Planets, Their Origin and Development, Yale University Press, New Haven, 1952 [ Links ]
9. KASTING, J. M., Origins of Life Evol. Biosphere, 1990, 20, 199. [ Links ]
10. DELSEMME, A. H., in CARLE, G., SCHWARTZ, D. and HUNTINGTON, J., (eds.), Exobiology in Solar System Exploration (NASA SP 512), 1992, p. 176. [ Links ]
11. HIROSE, Y., OHMURO, K., SAIGON, M., NAKAYAMA, T. and YAMAGATA Y., Origins of Life Evol. Biosphere, 1990, 20, 471.
12. BAR–NUN, A. and CHANG, S., J. Geophys. Res., 1983, 88, 6662.
13. CARLE, G., SCHWARTZ, D. and HUNTINGTON, J. (Eds.) Exobiology in Solar System Exploration, (NASA SP 512), 1992. [ Links ]
14. CHYBA, C. and SAGAN C., Nature, 1992, 355, 125.
15. KISSEL, J. and KRUEGER F. R., Nature, 1987, 326, 755.
16. KASAMATSU, T., KANEKO, T., SAITO, T. and KOBAYASHI, K., Bull. Chem. Soc. Jpn. 1997, 70, 1021.
17. KOBAYASHI, K., KANEKO, T., TSUCHIYA, M., SAITO, T., YAMAMOTO, T., KOIKE, T. and OSHIMA, T., Adv. Space. Res., 1995 a, 15, 127.
18. KOURIM, V., RAIS, J. and MILLION, B., J. Inorg. Nucl. Chem. 1964, 26, 1111.
19. TEWARI, B. B., MOHAN, D., KAMALUDDIN, SRIVASTAVA, S. K., Indian J. Chem. Technol. 1995, 2, 113. [ Links ]
20. FERRIS, J. P., Origins Life Evol. Biosphere, 1989, 19, 153. [ Links ]
21. ZHOU, G. and CHEN H. Anal. Chem, The Japan Society of Analytical Chemist, 2002, 18, 693