Introduction
The flower industry has become an important player in the global floriculture export market1. They are often used in vase displays, table decoration, garland, bouquet preparation, and various flower arrangements during religious ceremonies and social functions2. The vase life or postharvest longevity of cut flowers can be depicted as the prolonged existence of cut flowers in the vase while retaining their desirable qualities. It has great importance in the cut flower industry2. Postharvest life and quality of cut flowers can be affected by several factors described in three major categories, i.e., pre-harvest factors, harvest factors, and postharvest factors. The postharvest senescence directs to the short vase life of cut flowers and adversely affects cut flower marketing and commercialization3. The senescence of cut flowers is very complex with simultaneous changes. Notably, the studies on postharvest senescence physiology of cut flowers have been remarkably promoted and various techniques have been designed to slow down this process to improve the vase life of cut flowers thus, boost the profitability of floriculture producers4. Flowers require sincere, patient, soft, affectionate as well as expert handling5. All cut flowers are destined to die, and the challenge for postharvest researchers is to slow the processes controlling flower death to enable cut flowers to keep with a display life6.
All over the world, lily occupies a prominent place in horticulture as a cut flower, pot, and garden plan. However, postharvest senescence in the world, orchid occupies a prominent place in horticulture as a cut flower, pot, and garden plant6. As a cut flower, the orchid is now ranked as the fourth most important crop in the Netherlands. However, postharvest senescence is a major limitation to the marketing of many species of cut flowers been devoted to developing postharvest treatments to extend the marketing period of known that ethylene limitation to the marketing of many species of cut flower and considerable efforts have been devoted to developing postharvest treatments to extend the marketing period of them7.
It is well known that silver ion (applied as silver thiosulphate, STS) is widely used to delay senescence in ethylene-sensitive cut flowers because of its reducing ethylene-binding capacity and suppressing endogenous ethylene production (Van Doorn silver thiosulphate, STS) is widely used to sensitive cut flowers because the binding capacity and suppressing However, as a heavy metal salt and endogenous ethylene production8-10. Another ethylene inhibitor 1 methylcyclopropene (1-MCP), non-toxic to humans, has been demonstrated to extend the storage life of a range of cut flowers11 and potted flowering plants, and it is seen as an environmentally acceptable alternative to STS. Although 1-MCP can prevent some of the effects of ethylene, it often does so only for a short period12. An environment-friendly and more effective cut flower preservative should be exploited. friendly and more effective cut flower preservatives should be exploited. Nitric oxide (NO) was first characterized in plants in (1996)13 and has since been linked to a range of physiological and developmental signaling growth, development, and adaptive responses to multiple stresses, delays senescence modulation of endogenous ethylene. Postharvest application of NO by fumigation with NO gas has been shown effective in extending the postharvest life of a range of flowers, fruits, and vegetables when applied as a short-term fumigation treatment14. While NO and 1-MCP are alternative treatments to STS, the gaseous nature of both compounds has commercial operational knowledge, requires infrastructure, and is less suitable for developing countries. The importance of NO as a key regulator in mammalian physiology has led to substantial developments in NO donor technology, that is solid compounds that store NO chemically but release it under appropriate physical conditions15. A breakthrough in understanding the role of NO in plants relates to the identification of multiple, enzymatic as well as no enzymatic, NO generating systems, and widespread production, either constitutive or induced by biotic/abiotic stresses, of NO in plants16. Therefore, in the last decade, the role of NO in plants and agricultural production received much attention. Leshem & Wills14 stated that NO was a natural senescence-delaying plant growth-regulating agent acting primarily, but not solely, by down-regulating ethylene emission. Many studies have been done in this direction, but none of them have investigated the effect of sodium nitroprusside (SNP), which causes ethylene reduction and increases the lifespan of cut Dendrobium orchid flowers17-26.
This study explored this potential by examining the effects of exogenous NO on the vase life and the increase of fresh NO was first characterized in plants in 1996 and has since been linked to a range of physiological processes including signaling growth, development, and adaptive responses to multiple stresses, delays senescence and modulation ethylene27. Postharvest application of NO by fumigation with NO gas has been shown to affect postharvest28. The Orchid family (Orchidaceae) is the second largest family of flowering plants with approximately 30000 species with more than 880 genera29. This diversity increases towards the tropic; where the epiphytic species predominate that almost constitute 73 % of the family. Colombia is the country with the greatest number of species in America 3000, followed by Ecuador and Brazil 2500 each30. Germination of orchid seeds fully depends on a symbiotic association with soil-borne fungi, usually Rhizoctonia spp.31. In contrast to the peaceful symbiotic associations between many other terrestrial plants and mycorrhizal fungi, this association is a life-and-death struggle32. The fungi always try to invade the cytoplasm of orchid cells to obtain nutritional compounds. On the other hand, the orchid cells restrict the growth of the infecting hyphae and obtain nutrition by digesting them. Antifungal compounds are likely involved in the restriction of fungal growth33. Orchids have numerous varieties of exquisitely beautiful blossoms that are sold commercially, the only economically important product in this great plant family is the delicious spice known as vanilla34. Vanilla comes from several species of perennial vines of the genus Vanilla native to Mexico and tropical America. The Aztecs originally used vanilla as a flavoring for chocolate, and the Spanish conquerors carried it back to Europe where it was used for this same purpose35 One of the most famous ornamental orchids is the black orchid, Paphiopedilum wardii, this attractive species has light green leaves mottled dark green, with red-purple spotting on the underside of the leaf, which was first described in (1950)36 For hundreds of years’ orchid growers have been searching and hoping for a truly black orchid, as there is a certain fascination with black blooms that is rather hard to explain37. While some say that they have seen a black flower orchid, the general opinion is that this is false and that no orchid is truly black, but rather a deep blue or something of the sort, which may appear as black to the eye. The black flower orchid is then, in essence, nothing more than a myth38. Black flower orchid is also a name that is sometimes used for the Coelogyne flower39.
While NO and 1-MCP treatments for STS, the gaseous nature of both compounds increases commercial operational knowledge, requires infrastructure, and is less suitable for developing countries. The importance of NO as a key regulator in mammalian physiology has led to substantial developments in NO donor technology, which is a solid compound chemically released under appropriate physical conditions15. A breakthrough in understanding the role of NO in plants relates to the identification of multiple, enzymatic as well as no enzymatic, NO generating systems, production, either constitutive or induced by biotic/abiotic stresses, of NO in plants16. Therefore, in the past decade, the role of NO in plants and agricultural production received much attention. Leshem & Wills14 stated that NO was a natural senescence-delaying plant growth regulating agent acting primarily, but not solely, by down regular Badiyan et al.40. This study explored this potential by examining the effects of exogenous NO on the vase life and increase of free weight of orchid cut flower NO is a small diffusible and ubiquitous gaseous bioactive molecule and plays a variety of physiological and developmental roles in plants.
While NO and 1-MCP treatments for STS, the gaseous nature of both compounds increases commercial operational knowledge, requires infrastructure, and is less suitable for developing countries. The importance of NO as a key regulator in mammalian physiology has led to substantial developments in NO donor technology, which is a solid compound chemically released under appropriate physical conditions15. A breakthrough in understanding the role of NO in plants relates to the identification of multiple, enzymatic as well as no enzymatic, NO generating systems, production, either constitutive or induced by biotic/abiotic stresses, of NO in plants16. Therefore, in the past decade, the role of NO in plants and agricultural production received much attention. Leshem & Wills14 stated that NO was a natural senescence-delaying plant growth regulating agent acting primarily, but not solely, by down regular Badiyan et al.40. This study explored this potential by examining the effects of exogenous NO on the vase life and increase of free weight of orchid cut.
Materials and methods
Plant materials. Inflorescences of 5 commercial Dendrobium cultivars namely Planty Fushia (PF), Supica, Sakura, Milky Light, and New Anna were purchased from an export company (Thai Orchids Co., Ltd, Bangkok Thailand) with exporting grade40.
Screening ethylene-sensitive cultivars. Inflorescences of 5 Dendrobium were selected to be uniform, and their stems were cut underwater 150 mm from the last floret. The inflorescences were placed into 15 mL plastic tubes containing distilled water (control) or treated with 10 ppm ethphon for 6 h at room temperature (23±2º C)9. All treated inflorescences were kept under fluorescence light (12 µmol m-2 s-1) for 24 h. Vase life was evaluated as the time that opened flower and flower buds within inflorescences showed 50 % abscission. The sensitive cultivar was selected to evaluate the effect of SNP on the vase life41-43.
Effects of the concentrations of SNP on vase life of ethylene-sensitive cultivar. Dendrobium PF inflorescences were selected to investigate the effect of SNP on the vase life. Inflorescences were placed into 15 mL plastic tubes containing SNP solutions (Sigma, USA) at concentrations of 1, 2.5, 5, 10, 20, 40, and 80 µmol. L-1 under fluorescence light at 23±2º C for 24 h. The most effective concentration was used in further experiments44,45.
SNP treatment. In the previous experiment, it was observed that SNP concentration of 10 µmol L-1 was the optimum for long vase life, so it was selected to investigate the mechanism of prolongation of vase life of Dendrobium PF inflorescences compared to distilled water (control). All treatments were maintained at room temperature (23±2º C) under fluorescent lights for 24 h. Each treatment had 10 replicates15,46.
Ethylene production and respiration rate of inflorescence. Cut inflorescences were individually kept in 500 mL PVC-jar fitted with a silicone septum for 1 h, at ambient temperatures to determine ethylene production and respiration rate. Ethylene production was measured by gas chromatography using the method of Whitehead & Nelson47. A headspace gas sample (1 mL) was withdrawn using a gas-tight syringe and injected into a gas chromatograph (GC-14, Shimadzu, Japan) equipped with a Porapak Q 80/100 mesh column (Shimadzu) and a flame ionization detector. Results were expressed as µL C2H4 L-1 h-1. A second sample (1 mL) was withdrawn from the same jars to determine CO2 concentrations using a gas chromatograph (Shimadzu 8A, Japan). The respiration rate was calculated by following the method of Kader & Saltveit48 and the quantity was expressed as mg CO2 kg-1FW. h-1.
Lipid peroxidation. The level of lipid peroxidation in petal tissues was measured by the determination of malondialdehyde (MDA) content49-51. Petal samples (0.5 g) were homogenized in a cold homogenizer in 10 mL of 0.1 % trichloroacetic acid (TCA). The homogenate was centrifuged at 15000× g for 5 min. 4 µL of 0.5 % thiobarbituric acid in 20 % TCA was added to 2 mL of supernatant. The mixture was heated at 95º C for 30 min and then quickly cooled in an ice bath followed by centrifugation at 10000× g for 10 min. The MDA content in the supernatant was analyzed using a spectrophotometer at 532 nm. The concentrations of thiobarbituric acid reactive substances (TBARS) were calculated using an absorption coefficient of 155 mM-1 cm-1.
Membrane stability. Electrolyte leakage (EL) was measured by the method of another project with slight modifications. Segments (5 mm2) of petals were cut with a razor blade and washed in deionized water. Each 0.5 g sample was placed in an Erlenmeyer flask containing 30 mL of 0.3 M mannitol. The flasks were then shaken at 100 rpm for 1 h. The electro-conductivity of the solution was measured using a conductivity meter (Conductometer Consort, model C831, Turnhout, Belgium). Maximum conductance was measured after incubating the flasks in an autoclave at 121º C for 30 min followed by cooling to 25º C. Data were expressed as the percentage of maximum conductivity. 3 replicates were used per treatment. The electrolyte leakage was calculated by using the formula:
The number of opened buds was counted as the percentage of bud opening. Each treatment consists of 10 inflorescences.
ACC synthesis (ACS) activity. Extracts for ACS activity assay were prepared according to the method52-54 with slight modifications. 3 g of frozen petals or buds were homogenized in 6 mL of tricine extraction buffer (8.96 g of tricine in 250 mL water) containing 30 mg of polyvinyl pyrrolidone at pH 8.5. The homogenate was centrifuged at 15000× g at 4º C for 30 min. All steps in the enzyme extraction were carried out at 4º C. ACS activity was assayed in a vial using a reaction mixture containing 1.5 mL extract, 150 µL tricine reaction buffer, and 150 µL SAM chloride incubated at 25º C for 2 h with gentle shaking. Finally, 200 µL HgCl2 solution was added to stop the reaction then 20 µL of 50 µM ACC was added to the solution. The vial was then sealed with a cap. A 0.2 mL aliquot of NaOH-NaOCl was injected into the vial and mixed with the vortex mixture, then incubated for 4 min in an iced box. A 1 mL gas sample was taken from the headspace and injected into a gas chromatograph (GC-14, Shimadzu, Japan). The ACS activity was expressed as nL C2H4 g-1 FW h-1. All measurements were performed with 5 replications.
ACC oxidase (ACO) activity. Aliquots (1 g) of tissue were homogenized in a mortar and pestle with 3 mL extraction buffer [1 % (w/v) polyvinyl polypyrrolidone, 0.1 mM tricine (pH 7.5), 10 % glycerol, 5 mM dithiothreitol, and 30 mM sodium ascorbate] for 2 min. The homogenates were then centrifuged at 15000 × g for 30 min and the supernatants were collected for enzyme assay and placed in a gas-tight sample vial. All steps in the enzyme extraction were carried out at 4º C or on ice. After 1 h with gentle shaking at 30º C, 1 mL of headspace gas was withdrawn and monitored ethylene using a gas chromatograph (GC-14, Shimadzu, Japan). ACC oxidase activity was expressed as nL C2H4 g-1 FW h-1. 5 replications were used.
Statistical analysis. Experiments were performed using a completely randomized design (CRD). Data were analyzed using analysis of variance (ANOVA) in IBM SPSS Statistics version 25 (IBM Corporation, Armonk, NY, United States). Significant differences among the means ± standard error (SE) were declared at multiple comparisons via Duncan’s Multiple Range Test (DMRT) at P≤0.05. Charts and graphs are drawn through Table Pad Prism version 8.
Results
Effects of Genotypes and SNP concentrations on vase life of Dendrobium inflorescence. The application of 10 ppm ethephon significantly reduced the vase life of all tested cultivars. The vase life of Dendrobium PF, Dendrobium ‘Supica’, and Dendrobium ‘New Sakura’ decreased by more than 50 % compared to that of the control treatment. Dendrobium PF was the most sensitive cultivar and had the shortest vase life of 7 days after ethephon treatment compared to the control (22 days) (Figure 1).

Figure 1 Effect of ethylene treatment on the vase life of different Dendrobium cultivars treated with 10 ppm ethephon for 6 h at room temperature. All values are means ± SE of 10 replications and asterisks indicate significant differences between treatments at the Duncan's Multiple Range Test (DMRT) at 5 % level
Effects of SNP Concentrations on the vase life. Inflorescences of Dendrobium PF treated with 5 and 10 µmol L-1 SNP showed significantly longer vase life (20.1 and 22.7 days, respectively) compared with the control inflorescence (18 days). Therefore, the low concentrations of 5 and 10 µmol L-1 SNP treatment prevented floret abscission and bud drop. The high concentrations of SNP (>10 µmol L-1) significantly reduced the vase life compared to that of the control (Figure 2).

Figure 2 Vase life of Dendrobium 'Planty Fushia' inflorescences treated with different concentrations of SNP. All values are means ±SE of 10 replications and comparisons were made using Duncan's Multiple Range Test (DMRT) different letters indicate significant differences between treatments at the 5 % level
Effect of SNP on the percentage of bud opening. The bud opening increased during the vase life period (Figure 3). SNP-treated inflorescences showed a higher percentage of bud opening than the control and a significant difference from day 9 until the end of vase life. The untreated inflorescences (control) had 65 % open buds at the end of their vase life, while those treated with 10 µmol L-1 SNP showed almost 88 % open buds during vase life.
Effect of SNP on ethylene production and respiration rate. Ethylene production rates in the control treatment (treated with distilled water only) increased rapidly, the ethylene production sharply increased from day 1 to day 3 and reached the peak on Day 9 and then remained high until Day 9 (0.607±0.024 µL C2H4 L-1 h-1). In contrast, in the SNP, from day 1 to day 3 ethylene production smoothly increased and did not sharp incline as a control so didn't reach the highest rate until day 13 (0.471±0.019 µL C2H4 L-1 h-1) then declined slightly and this trend was significantly lower than the controls from day 3 onwards (Figure 4A). At day 9th (highest ethylene production in control) SNP treatment cause to 33 % reduction in ethylene production (0.61±0.024 to 0.41 ±0.031 µL C2H4 L-1 h-1 respectively) at the maximum amount on day 13th. Similar trends and results were seen in respiration rates (Figure 4B). An initial sharp increase in respiration occurred for 5 days in the controls and for 3 days in the SNP treatment. But the amount of respiration rate only in the beginning didn’t show a significant difference After these times, the respiration rates of both treatments plateaued, and the respiration rates of the SNP-treated inflorescences were significantly lower than that of the control. The maximum amount for the control group was observed on day 13th (48.61± 0.37 mg CO2 kg-1FW h-1) and in SNP treatment on day 9 (42.17± 0.49 mg CO2 kg-1 FW h-1).

Figure 3 Effect of SNP at 10 µM on bud opening of Dendrobium 'Planty Fushia' inflorescences. All values are means ±SE of 10 replications and asterisks indicate significant differences between treatments at the Duncan's Multiple Range Test (DMRT) at 5% level
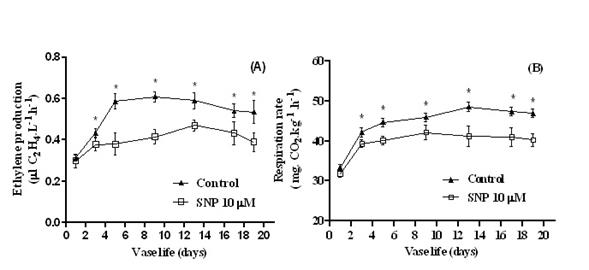
Figure 4 Changes of ethylene production (A) and respiration rate (B) of Dendrobium ‘Planty Fushia’ inflorescences treated with 10 µM of SNP in the vase solution (ð) and distilled water (control) (▲) during their vase life at 23±2 °C. All values are means ±SE of three replications and asterisks indicate significant differences between treatments at the Duncan's Multiple Range Test (DMRT) at 5% level

Figure 5 MDA content (A) and electrolyte leakage (B) of inflorescences of Dendrobium 'Planty Fushia' during their vase life at 23±2 °C. Values are means of three replications ± SE and asterisks indicate significant differences between treatments at the Duncan's Multiple Range Test (DMRT) at 5% level
Effect of SNP on lipid peroxidation and membrane stability. MDA contents and electrolyte leakage of both SNP-treated and control inflorescences increased throughout their vase lives (Figure 5A and 5B). However, MDA contents of inflorescences in the control treatment were higher than those in the SNP treatment from day 1 onwards; from day 3 onwards, meanwhile, electrolyte leakage had higher levels in the controls than in the inflorescences in the SNP treatment. The MDA content in SNP cut flowers treated increased from 0.7±0.11 µM g-1 FW to 1.1±0.23 µM g-1 FW during vase life but this amount was higher in the control group 0.76±0.15 µM g-1 FW to 1.26±0.26 µM g-1 FW during vase life (Figure 5A). According to MDA content in EL (%), we observed at the beginning hadn't a significant difference between control and SNP. However, on onward days always the quantity of EL in SNP-treated cut flowers was significantly lower than the control. In addition, the maximum amount of EL (%) was 52.14±1.12 and 49.02±0.83 respectively in control and SNP (Figure 5B). Our result indicated that in the initial days, SNP affected membrane permeability through the lower MDA content so it didn't show a sharp increase trend during the vase life of cut inflorescences (Figure 5A). Accordingly, lower MDA levels caused to have higher membrane stability (Figure 5B).

Figure 6 ACS activity in (A) buds and (B) opened flowers of inflorescences of Dendrobium ‘Planty Fushia’ during their vase life. Values are means of three replications ± SE and asterisks indicate significant differences between treatments at the 5% level
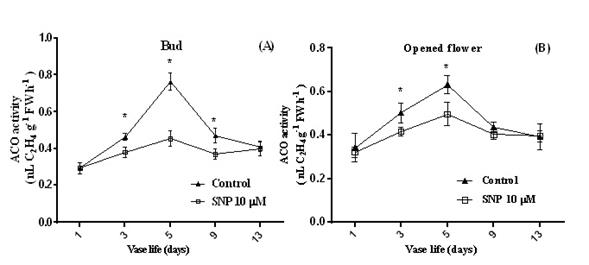
Figure 7 The ACO activity in (A) buds and (B) opened flowers of inflorescences of Dendrobium ‘Planty Fushia’ during their vase life. Values are means of three replications ± SE and asterisks indicate significant differences between treatments at the 5% level
Effect of SNP on ACC synthase and ACC oxidase activities. The ACC synthase (Figure 6) and ACC oxidase (Figure 7) activities of both SNP-treated and control at buds and open flowers tended to increase up to day 5 and then decrease. The activities of both enzymes in both floral stages were higher in the control than in those treated with SNP. ACC synthase and ACC oxidase activities deal with the capacity to convert ACC to ethylene were determined in SNP-treated cut flowers in floral buds and open flowers, delayed the rise in (and reduced the maximum of) ACC synthase and ACC oxidase activities in open flowers, and buds (Figures 6 and 7). According to our result (Figure 6B), SNP had a better effect on ACS activity in open flowers than buds so the trend of ACS activity slightly increased during vase life. Further, inflorescences treated with SNP (Figure 7A) in buds declared lower quantity and smooth increase in ACO activity than open flowers.
Discussion
Many studies have been done in this direction, in a study it was discussed how various factors affecting the efficacy of 1-MCP (such as concentration, treatment time and temperature, genotype, and flower stage) are involved in the achievement of flower longevity. Moreover, we highlight the advantages of applying nonvolatile and liquid 1-MCP formulation types, as opposed to using the conventional 1-MCP treatment (powder formulation type)55. Another study discusses how Lower temperatures markedly reduce both carbon dioxide concentration and ethylene production as well as its action. However, chilling-sensitive flowers, such as bird-of-paradise, heliconia, orchid, and ginger, cannot be stored below 10 to 13° C due to the intense development of tissue discoloration1. We expect that this review will provide useful information for the future utilization of 1-MCP for the maintenance of cut flower longevity. The senescence of cut flowers is a series of physiological and biochemical processes including changes in water balance, increase in hydrolytic enzyme activities, degradation of macromolecules, enhanced respiration activity, and loss of membrane integrity and cellular structure6,45,56,57. This study observed that supplying SNP in solution continuously enhanced the percentage of bud opening and increased flower longevity by reducing respiration rate, ethylene production, lipid peroxidation, and electrolyte leakage. The vase life of the 10 µmol L-1 SNP treatment was associated with lower ethylene production and lower respiration rate. The suppression of ethylene production in NO-treated cut flowers might be attributed to the suppression of ACO and ACS activities58-61. In this study, we also observed that the activities of ACS and ACO in both bud flower and opened flower were lower in SNP-treated inflorescences62. Similarly, found that carnations treated with 10 mg L-1 SNP suppress ethylene production14. Applying SNP reduced ethylene production in cut rose flowers15,18,63. The results showed that exogenous NO could significantly extend the vase life of cut lilium flowers and markedly increase fresh mass. Moreover, the results indicated that MB-1 could reverse the active effects of NO on different physiological indexes. Therefore, the vase life of cut lilium flowers was markedly extended by SNP treatment64. Another review shows that in this review, we summarize several potential approaches to improve flower vase life and discuss the best choices for holding-preservative-solution practices65.
It was observed that treating with SNP at 10 μmol L-1 effectively reduced the MDA content and electrolyte leakage in cut Dendrobium PF inflorescences. Lipid peroxidation is a complex process, which is normally initiated following the abstraction of a hydrogen atom from endogenous unsaturated fatty acids, resulting in the production of lipid radicals66,67. Increases in lipid peroxidation, usually determined from changes in MDA concentration, accompany the increase in Lysyl oxidase (LOX) activity while the products of peroxidation are considered to perturb membrane function. Therefore, MDA, a decomposition product of polyunsaturated fatty acids hydroperoxides, has been frequently described as a suitable biomarker for lipid peroxidation. NO might act as a strong scavenger of ROS that helps to better stability of plasma membrane integrity and cause lower levels of lipid peroxidation and electrolyte leakage in SNP treatment68,69.
However, 0.1 μmol L-1 SNP in Chrysanthemums and data declared prolongation of postharvest life because of NO strongly lowered cytochrome-mediate respiratory electron transport and cell respiration70.71. Also, it was the effect on the contributing functional activity of mitochondria72. Also observed was that by rising NO levels in strawberries respiration rate declined. The antioxidant activity of NO causes delayed senescence of plant tissues73. However, the Maintenance of antioxidants during vase life depended on genotype and the interaction between SNP dose and each cultivar. The role of SNPs in extending the vase life of some cut flowers by maintaining antioxidant activities has also been reported14. Furthermore, increased flower longevity is a result of decreased ethylene production by inhibiting both ACS and ACO activity. In other reports, although SNP in the vase solution promoted the abscission of open flowers, the younger buds continued to open even in the presence of high SNP concentrations. This review summarized some postharvest physiological changes in fresh-cut flowers, including moisture, respiratory metabolism, cell membranes, pigments, carbohydrate levels, and antioxidant systems, and how these physiological changes are affected by some exogenous substances. Although these compounds are widely used in the postharvest preservation of fresh-cut flowers and provide the basis for preserving other fresh-cut flowers, the preservation effect may vary by cultivar. Therefore, it is also necessary to create new, efficient, and popular preservation technologies to prolong the life of cut flowers and improve ornamental quality and economic benefits2.
In conclusion, this study has observed that the role of SNP, a NO donor, at 10 µmol L-1 might be as a signal molecule and be engaged in the ethylene response during the postharvest life of cut Dendrobium inflorescence. We of SNP on a vase of cut lilium flowers. They evaluated the effects of SNP on vase life and the increase of fresh weight of cut Lilium flowers. The results showed that exogenous NO could significantly extend the vase life of cut lilium flowers and markedly increase fresh mass. Moreover, the results indicated MB's ability to reverse the active effects of NO on different physiological indexes. Therefore, the vase life of cut flowers was markedly extended by SNP treatment. the vase life of cut lilium flowers and markedly increase fresh mass. Moreover, the results indicated that MB-1 could reverse the active effects of NO on different physiological indexes. Therefore, the vase life of cut lilium flowers was markedly extended by SNP treatment71. The results indicate that the SNP solution markedly affected the vase life of cut Dendrobium inflorescences, 10 µmol L-1 SNP could significantly increase bud opening and prolong the vase life compared with the control. In addition, the low concentration (10 µmol L-1) of SNP inhibited ethylene biosynthesis and suppressed respiration rate which correlated with low MDA content and low electrolyte leakage. Taken together, the data in this study confirm the positive role of SNP inhibits ethylene production by suppressing the key enzymes of ACS and ACO activities and enhancing the membrane stability resulting in extending the vase life of cut Dendrobium inflorescence. Thus, this is the first study using SNP as a NO donor in a vase solution with a cut orchid flower74. Germicides or biocides are commercially available anti-microbial chemicals that are intended to prevent the growth of bacteria, fungi, and other microorganisms in flower vases15,41,75-80.
A considerably further study will need to determine the mechanism of interaction among SNP and ethylene in orchid floral tissues, including the gene expression analyses and also a study of how antioxidants modulated the vase life of cut orchids.
Cut flowers are more perishable and under normal conditions they last only for a few days while maintaining their beauty and attractiveness. The relatively short postharvest life of most cut flowers has become a crucial issue in commercial cut flower production enterprises. Hence, improving the postharvest life of cut flowers has been promoted and an insightful understanding of several factors that determine the postharvest life of cut flowers is imperative to enhance the vase life of cut flowers. Thus, the present review insightfully depicted the key factors affecting on postharvest life of cut flowers and different approaches to extend the vase life.











 uBio
uBio